49800
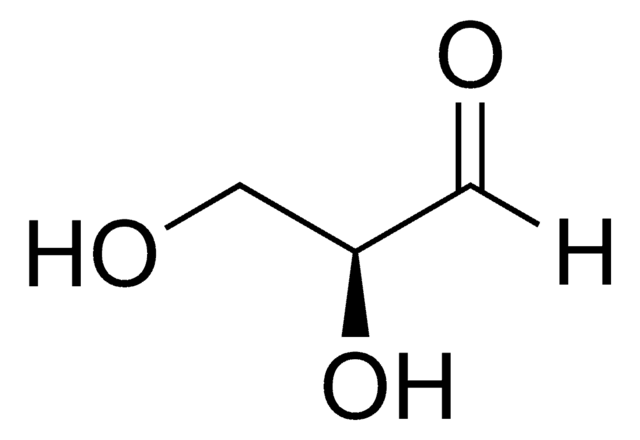
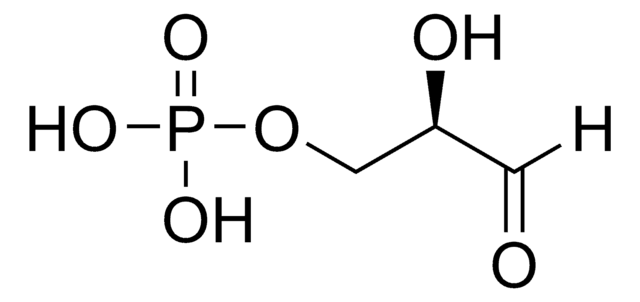
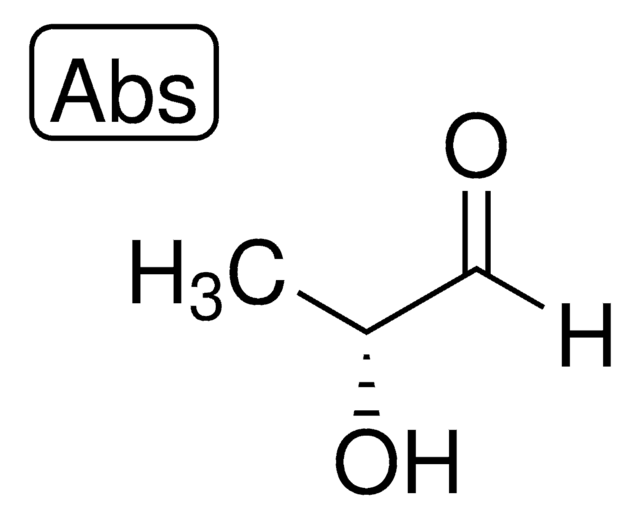
D-(+)-Glyceraldehyde
≥98.0% (HPLC)
Synonym(s):
(2R)-2,3-Dihydroxypropanal, Triose
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C3H6O3
CAS Number:
Molecular Weight:
90.08
Beilstein:
1720474
EC Number:
MDL number:
UNSPSC Code:
12352200
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
≥98.0% (HPLC)
impurities
≤10% water
storage temp.
2-8°C
SMILES string
OC[C@@H](O)C=O
InChI
1S/C3H6O3/c4-1-3(6)2-5/h1,3,5-6H,2H2/t3-/m0/s1
InChI key
MNQZXJOMYWMBOU-VKHMYHEASA-N
Looking for similar products? Visit Product Comparison Guide
Application
D-(+)-Glyceraldehyde can be utilized as a reactant in the synthesis of:
- (S)-homophenylalanine by ruthenium oxidation of a 3-amino-1,2-diol generated via coupling of an amine, and α-hydroxyaldehyde.
- β- and γ-allenols via metal-catalyzed cyclization. Allenols are used as a key precursor for the preparation of enantiopure dihydropyrans and tetrahydrooxepines.
- Isopropylidene D-glyceraldehyde intermediate, which controls the chirality in the total synthesis of prostaglandins (PGE1).
Storage Class Code
10 - Combustible liquids
WGK
WGK 3
Flash Point(F)
235.4 °F - closed cup
Flash Point(C)
113 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Highly stereocontrolled one-step synthesis of anti-β-amino alcohols from organoboronic acids, amines, and α-hydroxy aldehydes
Petasis NA and Zavialov IA
Journal of the American Chemical Society, 120(45), 11798-11799 (1998)
Chiral synthesis of prostaglandins (PGE1) from D-glyceraldehyde
Stork G and Takahashi T
Journal of the American Chemical Society, 99(4), 1275-1276 (1977)
Takayoshi Wakagi et al.
PloS one, 11(1), e0147333-e0147333 (2016-01-26)
Archaea use glycolytic pathways distinct from those found in bacteria and eukaryotes, where unique enzymes catalyze each reaction step. In this study, we isolated three isozymes of glyceraldehyde oxidoreductase (GAOR1, GAOR2 and GAOR3) from the thermoacidophilic archaeon Sulfolobus tokodaii. GAOR1-3
Katarzyna Lechowicz et al.
International journal of molecular sciences, 21(16) (2020-08-13)
Lolium multiflorum/Festuca arundinacea introgression forms have been proved several times to be good models to identify key components of grass metabolism involved in the mechanisms of tolerance to water deficit. Here, for the first time, a relationship between photosynthetic and
Metal-Catalyzed Cyclization of β-and γ-Allenols Derived from d-Glyceraldehyde- Synthesis of Enantiopure Dihydropyrans and Tetrahydrooxepines: An Experimental and Theoretical Study
Alcaide BL
Chemistry?A European Journal, 15(36), 9127-9138 (2009)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service