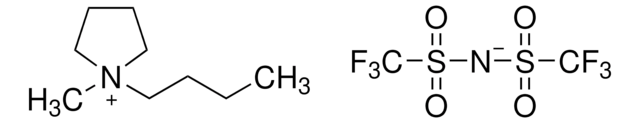449504
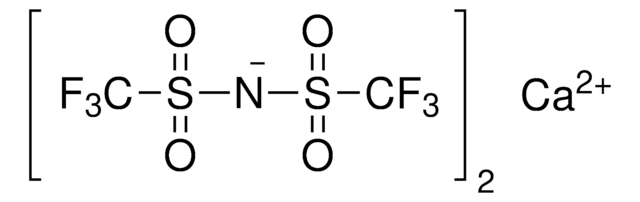
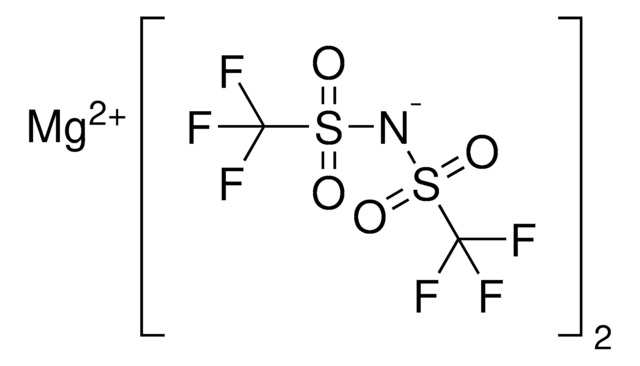
Bis(trifluoromethane)sulfonimide lithium salt
Synonym(s):
Bis(trifluoromethylsulfonyl)amine lithium salt, Lithium bistrifluoromethanesulfonimidate
About This Item
Recommended Products
grade
for analytical purposes
mp
234-238 °C (lit.)
functional group
fluoro
SMILES string
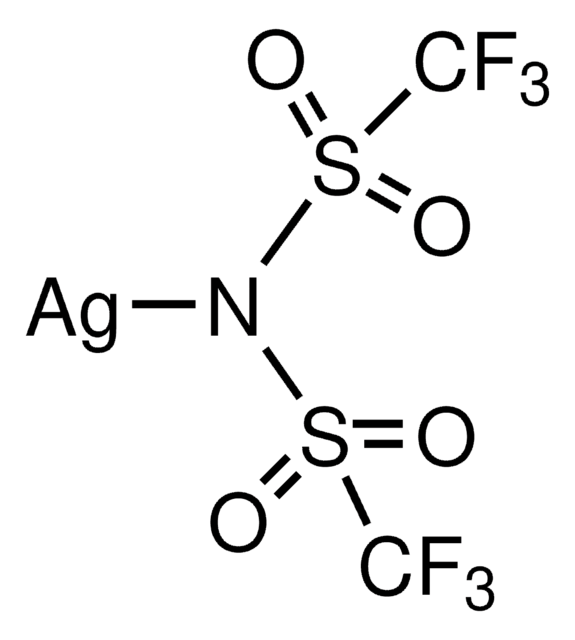
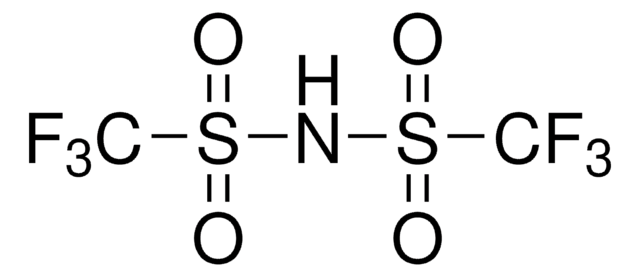
[Li]N(S(=O)(=O)C(F)(F)F)S(=O)(=O)C(F)(F)F
InChI
1S/C2F6NO4S2.Li/c3-1(4,5)14(10,11)9-15(12,13)2(6,7)8;/q-1;+1
InChI key
QSZMZKBZAYQGRS-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
Application
- As a chemical additive for improving the power conversion efficiencies in porphyrin-based organic solar cells.
- As a reagent in the preparation of imidazolium core bearing monomer ionic liquids to develop polymerized ionic liquids.
- For the preparation of a chiral imidazolium salt via anion metathesis of the corresponding triflate.
- In the synthesis of solid polymer electrolytes for lithium-ion batteries.
- In the synthesis of polyelectrolyte reusable homogenous catalysts, which are used in the Diels–Alder reactions between isoprene and a variety of dienophiles.
- Used in the preparation of electrolytes for lithium batteries and novel rare-earth Lewis acid catalysts.
Other Notes
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 3 Dermal - Acute Tox. 3 Oral - Aquatic Chronic 3 - Eye Dam. 1 - Skin Corr. 1B - STOT RE 2 Oral
Target Organs
Nervous system
Storage Class Code
6.1B - Non-combustible acute toxic Cat. 1 and 2 / very toxic hazardous materials
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Articles
Dr. Schmuch, Dr. Siozios, Professor Dr. Winter, and Dr. Placke review the challenges and opportunities of nickelrich layered oxide cathode materials. They discuss production processes for the layered oxide cathode materials as well as their chemistry and morphology.
The critical technical challenges associated with the commercialization of electric vehicle batteries include cost, performance, abuse tolerance, and lifespan.
Lithium-ion batteries (LIBs) have been widely adopted as the most promising portable energy source in electronic devices because of their high working voltage, high energy density, and good cyclic performance.
Due to the adverse impact of the continued use of fossil fuels on the earth’s environment and climate, researchers have been asked to develop new approaches for producing power using renewable sources like wind and solar energy
Global Trade Item Number
| SKU | GTIN |
|---|---|
| 449504-50G | 4061832323312 |
| 449504-10G | 4061832323305 |
| 449504-250G | 4065265906937 |
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service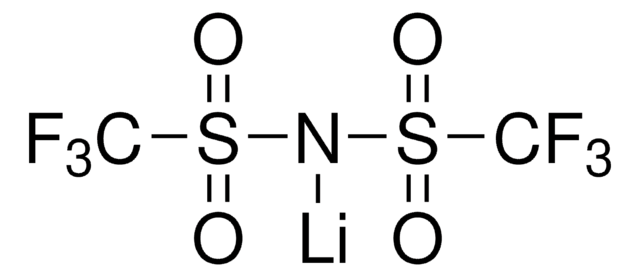



![Zinc di[bis(trifluoromethylsulfonyl)imide] 95%](/deepweb/assets/sigmaaldrich/product/structures/336/073/952daadd-0a7c-4bec-bbaf-442a24c62161/640/952daadd-0a7c-4bec-bbaf-442a24c62161.png)