334987
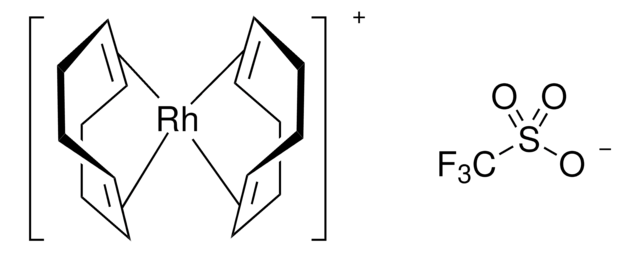
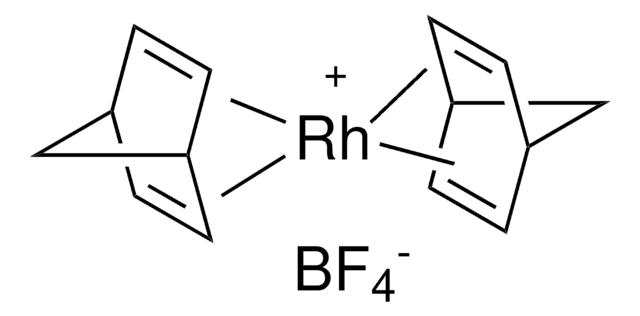
Bis(1,5-cyclooctadiene)rhodium(I) tetrafluoroborate hydrate
97%
About This Item
Recommended Products
Assay
97%
reaction suitability
core: rhodium
reagent type: catalyst
mp
165 °C (dec.) (lit.)
SMILES string
O.[Rh+].F[B-](F)(F)F.C1CC=CCCC=C1.C2CC=CCCC=C2
InChI
1S/2C8H12.BF4.H2O.Rh/c2*1-2-4-6-8-7-5-3-1;2-1(3,4)5;;/h2*1-2,7-8H,3-6H2;;1H2;/q;;-1;;+1/b2*2-1-,8-7-;;;
InChI key
NYMNQNLSUITCLM-DIURZKMRSA-N
Looking for similar products? Visit Product Comparison Guide
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Flam. Sol. 2 - Skin Irrit. 2 - Skin Sens. 1 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
4.1B - Flammable solid hazardous materials
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Articles
Vinyl-metal reagents play a pivotal role in organic synthesis. Among the vinyl-metal reagents available, silicon-based reagents are of increasing importance. This is largely due to their low cost, minimal toxicity, ease of handling, and the simplicity of byproduct removal.
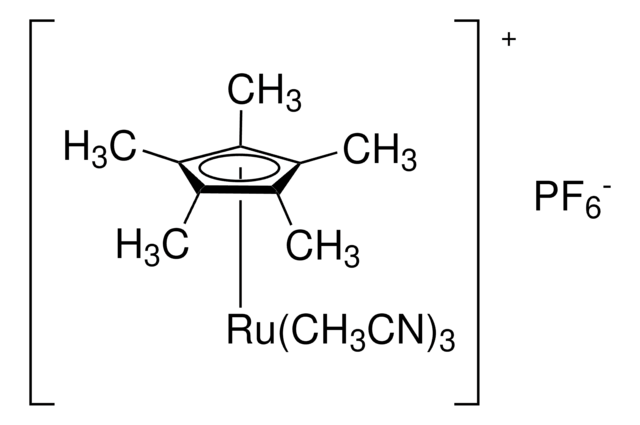
This robust protocol for hydrosilylation of terminal acetylenes to give α-vinylsilanes using [Cp*Ru(MeCN)3]PF6, as well as a number of other catalysts for hydrosilylation, comes from the Trost group at Stanford University.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service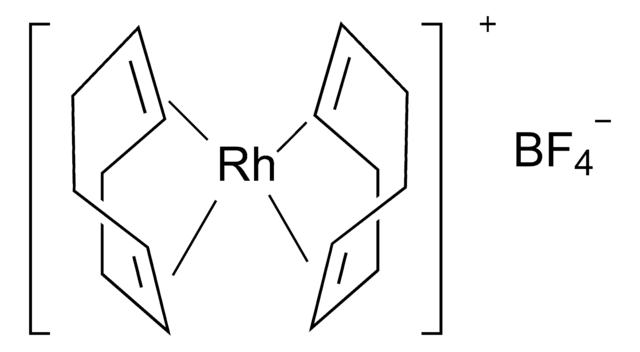

![RuCl(p-cymene)[(S,S)-Ts-DPEN]](/deepweb/assets/sigmaaldrich/product/structures/596/849/f8e3d2d8-a02e-430e-b0ed-c208cea3a6fb/640/f8e3d2d8-a02e-430e-b0ed-c208cea3a6fb.png)