All Photos(3)
About This Item
Empirical Formula (Hill Notation):
C10H11NO2
CAS Number:
Molecular Weight:
177.20
Beilstein:
4782551
MDL number:
UNSPSC Code:
12352005
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
99%
form
powder
optical activity
[α]18/D +64°, c = 1 in chloroform
mp
88-90 °C
SMILES string
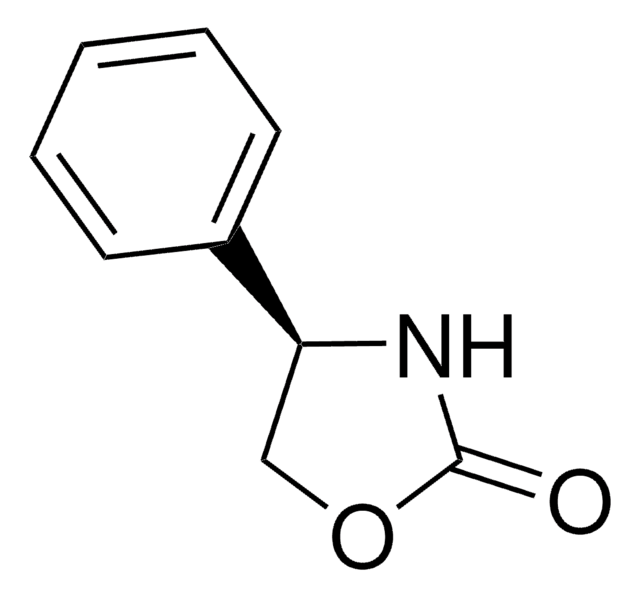
O=C1N[C@@H](CO1)Cc2ccccc2
InChI
1S/C10H11NO2/c12-10-11-9(7-13-10)6-8-4-2-1-3-5-8/h1-5,9H,6-7H2,(H,11,12)/t9-/m1/s1
InChI key
OJOFMLDBXPDXLQ-SECBINFHSA-N
Looking for similar products? Visit Product Comparison Guide
Application
(R)-4-Benzyl-2-oxazolidinone may be used as a starting material in the synthesis of enantiopure carbocyclic nucleosides. It may also be used as a chiral auxiliary in the enantioselective synthesis of (2R,2′S)-erythro-methylphenidate.
Used in the synthesis of HIV protease inhibitors.
Versatile chiral auxiliary for asymmetric synthesis. For a recent review, see Aldrichimica Acta.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Synlett, 4, 679-683 (2004)
Enantioselective synthesis of (2S, 2' R)-erythro-methylphenidate.
Prashad M, et al.
Tetrahedron Asymmetry, 10(18), 3479-3482 (1999)
An efficient asymmetric approach to carbocyclic nucleosides: asymmetric synthesis of 1592U89, a potent inhibitor of HIV reverse transcriptase.
Crimmins MT and King BW.
The Journal of Organic Chemistry, 61, 4192-4193 (1996)
Ager, D.J., et al.
Aldrichimica Acta, 30, 3-3 (1997)
Chromatograms
application for HPLCOur team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service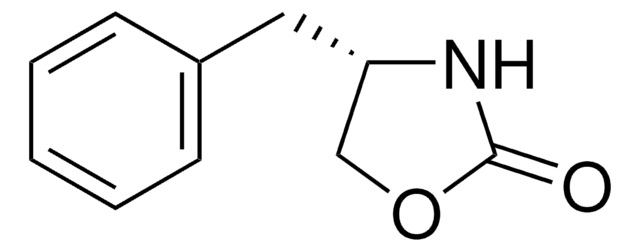

![1,8-Diazabicyclo[5.4.0]undec-7-ene 98%](/deepweb/assets/sigmaaldrich/product/structures/120/564/5b373e23-1624-489c-8efb-692de0f96ffb/640/5b373e23-1624-489c-8efb-692de0f96ffb.png)