292818
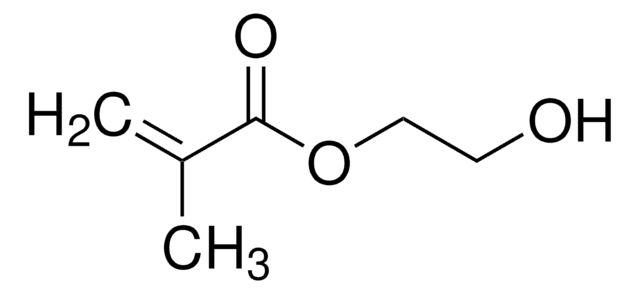
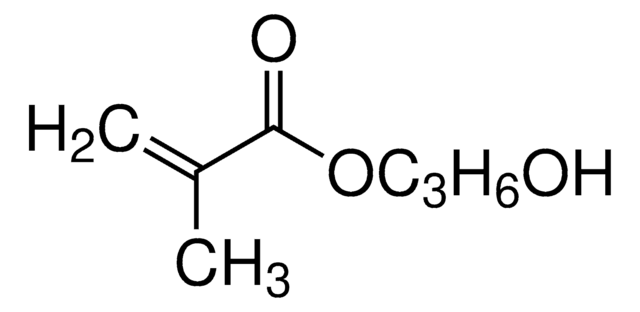
2-Hydroxyethyl acrylate
96%, contains 200-650 ppm monomethyl ether hydroquinone as inhibitor
Synonym(s):
Ethylene glycol monoacrylate
About This Item
Recommended Products
vapor density
>1 (vs air)
Quality Level
vapor pressure
<0.1 mmHg ( 20 °C)
Assay
96%
form
solid
contains
200-650 ppm monomethyl ether hydroquinone as inhibitor
refractive index
n20/D 1.45 (lit.)
bp
90-92 °C/12 mmHg (lit.)
density
1.011 g/mL at 25 °C (lit.)
storage temp.
2-8°C
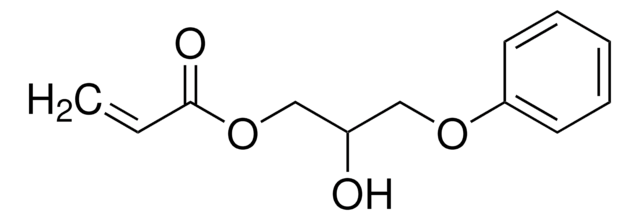
SMILES string
OCCOC(=O)C=C
InChI
1S/C5H8O3/c1-2-5(7)8-4-3-6/h2,6H,1,3-4H2
InChI key
OMIGHNLMNHATMP-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
Application
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 3 Dermal - Acute Tox. 4 Oral - Aquatic Acute 1 - Aquatic Chronic 3 - Eye Dam. 1 - Skin Corr. 1B - Skin Sens. 1
Storage Class Code
6.1A - Combustible acute toxic Cat. 1 and 2 / very toxic hazardous materials
WGK
WGK 3
Flash Point(F)
213.8 °F - closed cup
Flash Point(C)
101 °C - closed cup
Personal Protective Equipment
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Articles
The manufacture of monomers for use in ophthalmic applications is driven by the need for higher purity, improved reliability of manufacturing supply, but ultimately by the need for the increased comfort, convenience, and safety of contact lens wearers. Daily wear contact lenses have the potential to fill this need for many customers; however, their widespread use is constrained by higher costs compared to weekly- or monthly-based lenses. New approaches that improve cost structure and result in higher quality raw materials are needed to help make contact lenses more affordable and accelerate growth of the contact lens market.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service