256269
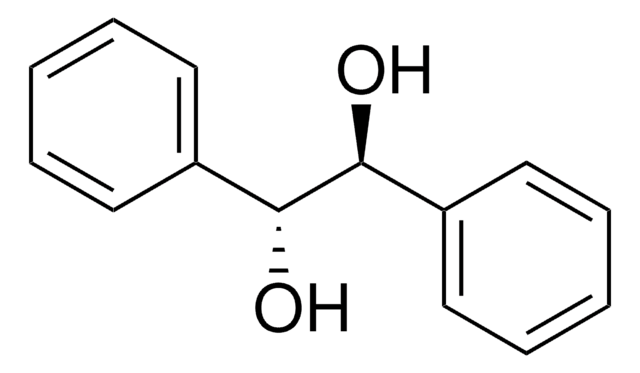
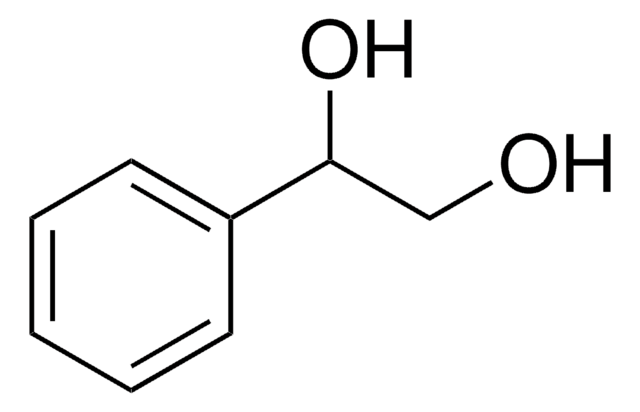
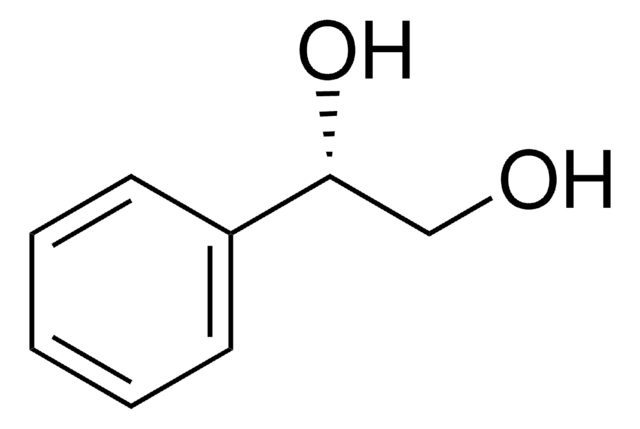
(S,S)-(−)-Hydrobenzoin
99%, optical purity ee: 99% (GLC)
Synonym(s):
(S,S)-(−)-1,2-Diphenyl-1,2-ethanediol, (S,S)-1,2-Diphenylethylene glycol
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Linear Formula:
C6H5CH(OH)CH(OH)C6H5
CAS Number:
Molecular Weight:
214.26
Beilstein:
2330888
MDL number:
UNSPSC Code:
12352002
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
99%
optical activity
[α]24/D −94°, c = 2.5 in ethanol
optical purity
ee: 99% (GLC)
mp
148-150 °C (lit.)
SMILES string
O[C@H]([C@@H](O)c1ccccc1)c2ccccc2
InChI
1S/C14H14O2/c15-13(11-7-3-1-4-8-11)14(16)12-9-5-2-6-10-12/h1-10,13-16H/t13-,14-/m0/s1
InChI key
IHPDTPWNFBQHEB-KBPBESRZSA-N
Looking for similar products? Visit Product Comparison Guide
General description
Chiral reagent.
Application
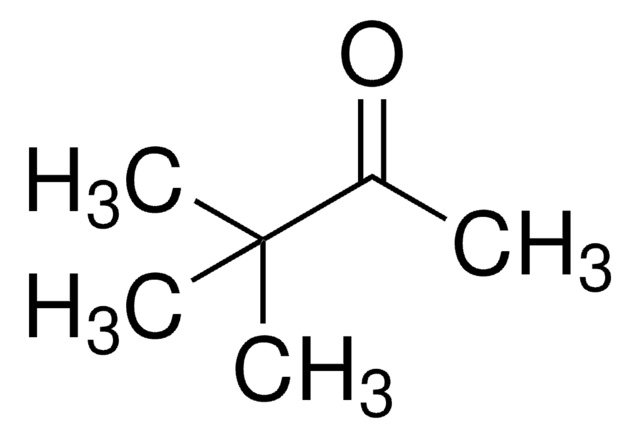
(S,S)-(-)-Hydrobenzoin may also be used to prepare (1S,2S,1′S)- and (1S,2S,1′R)-2-(cyclohex-2′-enyloxy)-1,2-diphenylethanol, which are intermediates to prepare enantiopure cyclohexitols. The (S,S)-(-)-hydrobenzoin/Ca complex may be used to catalyze the direct asymmetric aldol reaction of acetophenone and pivalaldehyde to form (R)-3-hydroxy-4,4-dimethyl-1-phenylpentan-1-one.
C2 symmetric chiral diol with versatile applications as a chiral auxiliary, building block, and chiral ligand.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Catalytic asymmetric aldol reaction of ketones and aldehydes using chiral calcium alkoxides.
Tetrahedron Letters, 42(28), 4669-4671 (2001)
Synthesis of enantiopure cyclitols from (?)-3-bromocyclohexene mediated by intramolecular oxyselenenylation employing (S, S)-hydrobenzoin and (S)-mandelic acid as chiral sources.
Lee YJ, et al.
Tetrahedron, 61(8), 1987-2001 (2005)
Marshall, J.A. Xie, S.
The Journal of Organic Chemistry, 60, 7230-7230 (1995)
Stolle, A. et al.
Tetrahedron Letters, 35, 3521-3521 (1994)
Pichon, M. Figadere, B.
Tetrahedron Asymmetry, 7, 927-927 (1996)
Chromatograms
application for HPLCOur team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service