249939
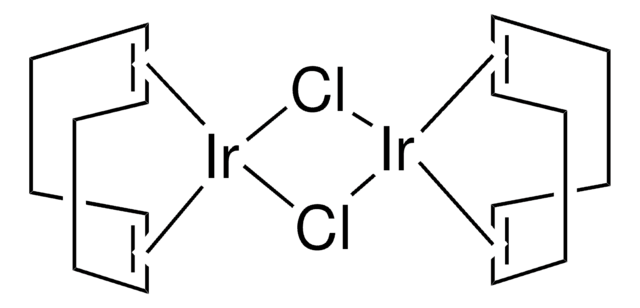
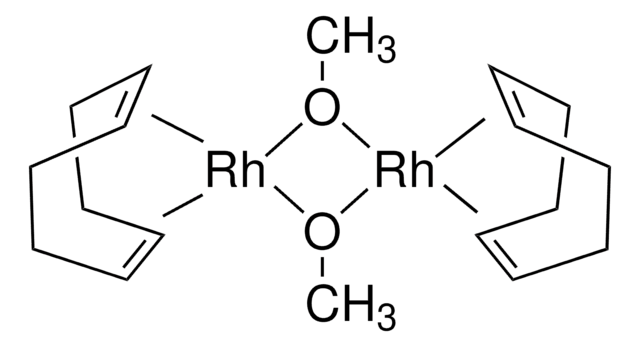
Bicyclo[2.2.1]hepta-2,5-diene-rhodium(I) chloride dimer
96%
Synonym(s):
[Rh(nbd)Cl]2, dichlorobis(norbornadiene)dirhodium, 2,5-Norbornadiene-rhodium(I) chloride dimer, Chloro(2,5-norbornadiene)rhodium(I) dimer, Rhodium(I) chloride 2,5-Norbornadiene complex dimer, [Rh(nbd)Cl]2
About This Item
Recommended Products
Quality Level
Assay
96%
form
powder
reaction suitability
core: rhodium
reagent type: catalyst
SMILES string
Cl[Rh].Cl[Rh].[CH]1[CH]C2[CH][CH]C1C2.[CH]3[CH]C4[CH][CH]C3C4
InChI
1S/2C7H8.2ClH.2Rh/c2*1-2-7-4-3-6(1)5-7;;;;/h2*1-4,6-7H,5H2;2*1H;;/q;;;;2*+1/p-2/t2*6-,7+;;;;
InChI key
RXDWVIOULPVOEO-SEGRDSFWSA-L
Looking for similar products? Visit Product Comparison Guide
Related Categories
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Articles
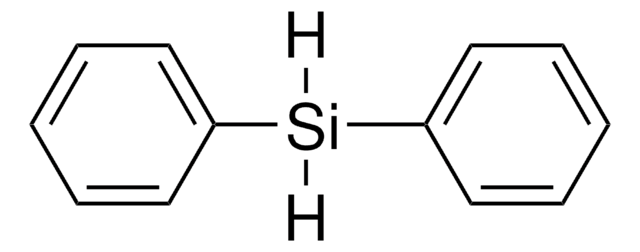
Vinyl-metal reagents play a pivotal role in organic synthesis. Among the vinyl-metal reagents available, silicon-based reagents are of increasing importance. This is largely due to their low cost, minimal toxicity, ease of handling, and the simplicity of byproduct removal.
This robust protocol for hydrosilylation of terminal acetylenes to give α-vinylsilanes using [Cp*Ru(MeCN)3]PF6, as well as a number of other catalysts for hydrosilylation, comes from the Trost group at Stanford University.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service
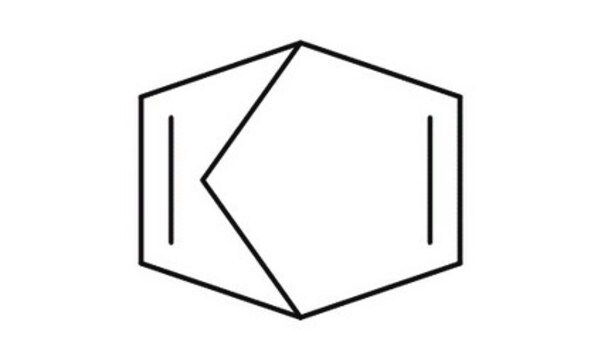
![Bicyclo[2.2.1]hepta-2,5-diene 98%](/deepweb/assets/sigmaaldrich/product/structures/304/819/dfa7c176-c370-4fb5-acf1-28d751241a50/640/dfa7c176-c370-4fb5-acf1-28d751241a50.png)
rhodium(I) tetrafluoroborate 98%](/deepweb/assets/sigmaaldrich/product/structures/138/264/047825b4-1f5a-486a-9f51-8d9c18b1382f/640/047825b4-1f5a-486a-9f51-8d9c18b1382f.png)