All Photos(1)
About This Item
Linear Formula:
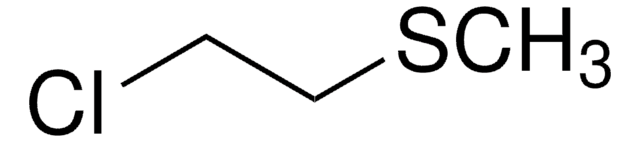
ClCH2CH2SC2H5
CAS Number:
Molecular Weight:
124.63
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
97%
form
liquid
refractive index
n20/D 1.4885 (lit.)
bp
156-157 °C (lit.)
density
1.07 g/mL at 25 °C (lit.)
functional group
chloro
thioether
storage temp.
2-8°C
SMILES string
CCSCCCl
InChI
1S/C4H9ClS/c1-2-6-4-3-5/h2-4H2,1H3
InChI key
GBNVXYXIRHSYEG-UHFFFAOYSA-N
Related Categories
General description
2-Chloroethyl ethyl sulfide is a monofunctional analog of sulfur mustard (SM; 2,2′-dichloro diethyl sulfide). The mass diffusivity of 2-chloroethyl ethyl sulfide, a chemical warfare agent simulant, was studied.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 3 Dermal - Acute Tox. 3 Inhalation - Acute Tox. 3 Oral - Carc. 1A - Eye Dam. 1 - Flam. Liq. 3 - Skin Corr. 1B
Storage Class Code
3 - Flammable liquids
WGK
WGK 3
Flash Point(F)
125.6 °F - closed cup
Flash Point(C)
52 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Adrienne T Black et al.
Toxicology and applied pharmacology, 249(2), 178-187 (2010-09-16)
Sulfur mustard is a potent vesicant that induces inflammation, edema and blistering following dermal exposure. To assess molecular mechanisms mediating these responses, we analyzed the effects of the model sulfur mustard vesicant, 2-chloroethyl ethyl sulfide, on EpiDerm-FT™, a commercially available
Heidi C O'Neill et al.
Free radical biology & medicine, 48(9), 1188-1196 (2010-02-09)
Sulfur mustard (bis-2-(chloroethyl) sulfide; SM) is a highly reactive vesicating and alkylating chemical warfare agent. A SM analog, 2-chloroethyl ethyl sulfide (CEES), has been utilized to elucidate mechanisms of toxicity and as a screen for therapeutics. Previous studies with SM
Tabea Zubel et al.
Archives of toxicology, 93(1), 61-79 (2018-10-17)
Despite its worldwide ban, the warfare agent sulfur mustard (SM) still represents a realistic threat, due to potential release in terroristic attacks and asymmetric conflicts. Therefore, the rigorous and quantitative detection of SM exposure is crucial for diagnosis, health risk
Swetha Inturi et al.
Free radical biology & medicine, 51(12), 2272-2280 (2011-09-17)
Employing mouse skin epidermal JB6 cells and dermal fibroblasts, here we examined the mechanisms of DNA damage by 2-chloroethyl ethyl sulfide (CEES), a monofunctional analog of sulfur mustard (SM). CEES exposure caused H2A.X and p53 phosphorylation as well as p53
Joshua P Gray et al.
Toxicology and applied pharmacology, 247(2), 76-82 (2010-06-22)
Inhalation of vesicants including sulfur mustard can cause significant damage to the upper airways. This is the result of vesicant-induced modifications of proteins important in maintaining the integrity of the lung. Cytochrome P450s are the major enzymes in the lung
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service