All Photos(1)
About This Item
Empirical Formula (Hill Notation):
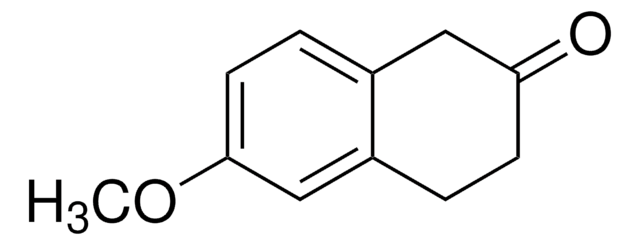
C10H10O2
CAS Number:
Molecular Weight:
162.19
Beilstein:
2437410
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
99%
mp
206-209 °C (lit.)
functional group
ketone
SMILES string
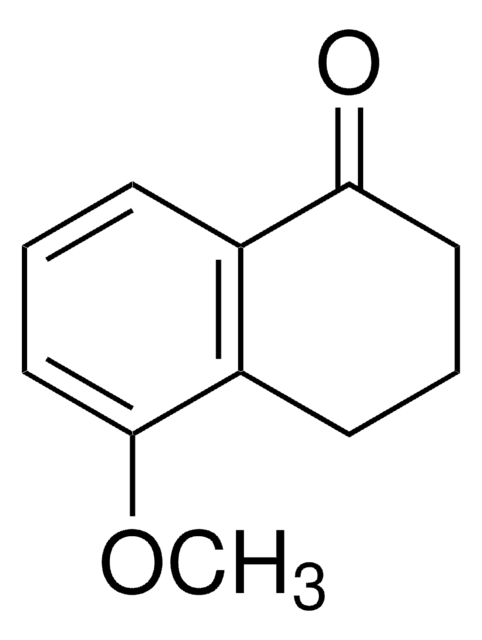
Oc1cccc2C(=O)CCCc12
InChI
1S/C10H10O2/c11-9-5-1-3-7-8(9)4-2-6-10(7)12/h1,3,5,11H,2,4,6H2
InChI key
YPPZCRZRQHFRBH-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
5-Hydroxy-1-tetralone was used:
- as internal standard during HPLC determination of 4-hydroxymephenytoin (4-OH-M) in human urine
- as fluorescent labeling reagent during microdetection of glycosphingolipid on TLC plates
- in synthesis of 1,2,3,4-tetrahydro-5H-1-benzazepine-quinone derivatives
- in synthesis of new chiral oxathiane
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
A new chiral oxathiane: synthesis, resolution and absolute configuration determination by vibrational circular dichroism.
Solladie-Cavallo A, et al.
Tetrahedron Asymmetry, 12(18), 2605-2611 (2001)
K Watanabe et al.
Journal of lipid research, 36(8), 1848-1855 (1995-08-01)
A microdetection system for glycosphingolipid analysis has been developed using 5-hydroxy-1-tetralone as the fluorescent labeling reagent. The reagents in H2SO4 permit the fluorometric detection of acidic and neutral glycosphingolipids both in test tube and on thin-layer chromatographic plates. Glycosphingolipids can
Studies on Quinones. Part 22.'Synthesis of 1-Benzazepine-6, 9-Quinone Derivatives.
Valderrama JA, et al.
Synthetic Communications, 22(4), 629-640 (1992)
M Tanaka et al.
Journal of chromatography. B, Biomedical applications, 676(1), 87-94 (1996-02-09)
A simple and selective HPLC method for the determination of 4-hydroxymephenytoin (4-OH-M) in human urine, using a controlled potential coulometric detector equipped with a dual working electrode cell of fully porous graphite, has been developed. After acid hydrolysis of urine
Global Trade Item Number
| SKU | GTIN |
|---|---|
| 219975-1G | |
| 219975-5G | 4061831810127 |
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service