All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C6H9NO2
CAS Number:
Molecular Weight:
127.14
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
99%
form
liquid
refractive index
n20/D 1.505 (lit.)
bp
66-68 °C/1.5 mmHg (lit.)
density
1.127 g/mL at 25 °C (lit.)
functional group
nitro
storage temp.
2-8°C
SMILES string
[O-][N+](=O)C1=CCCCC1
InChI
1S/C6H9NO2/c8-7(9)6-4-2-1-3-5-6/h4H,1-3,5H2
InChI key
DJBRXNRKYAWTBL-UHFFFAOYSA-N
General description
Chemoselective hydrogenation of 1-nitro-1-cyclohexene catalyzed by gold nanoparticles supported on TiO2 or Fe2O3 has been reported.
Application
1-Nitro-1-cyclohexene was used in the preparation of substituted anilines via chemoselective hydrogenation catalyzed by supported gold nanoparticles (Au/TiO2 and Au/Fe2O3).
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2
Storage Class Code
10 - Combustible liquids
WGK
WGK 3
Flash Point(F)
174.2 °F - closed cup
Flash Point(C)
79 °C - closed cup
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Avelino Corma et al.
Nature protocols, 1(6), 2590-2595 (2007-04-05)
A protocol for the chemoselective hydrogenation of nitro compounds to the corresponding anilines by means of supported gold catalysts is described. Nitro groups on different compounds--containing double bonds, carbonyl, nitrile or amide groups--have been successfully hydrogenated on supported gold nanoparticles
Avelino Corma et al.
Science (New York, N.Y.), 313(5785), 332-334 (2006-07-22)
The selective reduction of a nitro group when other reducible functions are present is a difficult process that often requires stoichiometric amounts of reducing agents or, if H2 is used, the addition of soluble metals. Gold nanoparticles supported on TiO2
Fabian Niedermair et al.
Inorganic chemistry, 49(20), 9333-9342 (2010-09-16)
A series of π-extended phosphorescent palladium(II) and platinum(II) porphyrin complexes were synthesized, in which additional benzene rings are fused radially onto at least one of the four peripheral benzo groups. The photophysical properties of the metalloporphyrins palladium(II)-meso-tetra-(4-fluorophenyl)mononaphthotribenzoporphyrin (Pd1NF), cis-palladium(II)-meso-tetra-(4-fluorophenyl)dibenzodinaphthoporphyrin (Pd2NF)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service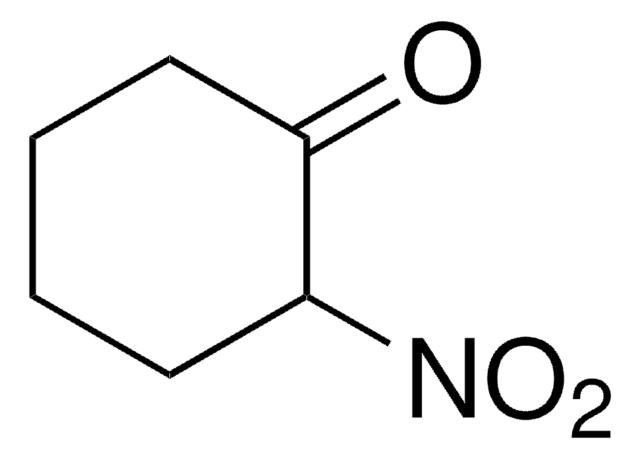


![[1,2-Bis(diphenylphosphino)ethane]dichloronickel(II)](/deepweb/assets/sigmaaldrich/product/structures/707/956/483e7a6e-5fb5-4e39-abd1-ecf33ccab3cf/640/483e7a6e-5fb5-4e39-abd1-ecf33ccab3cf.png)