All Photos(1)
About This Item
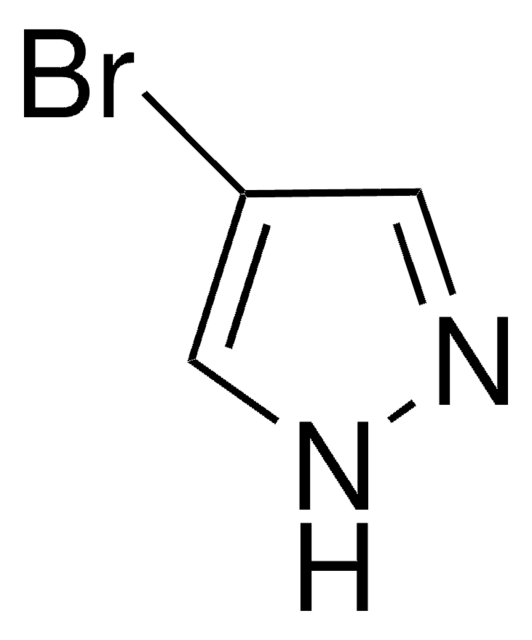
Empirical Formula (Hill Notation):
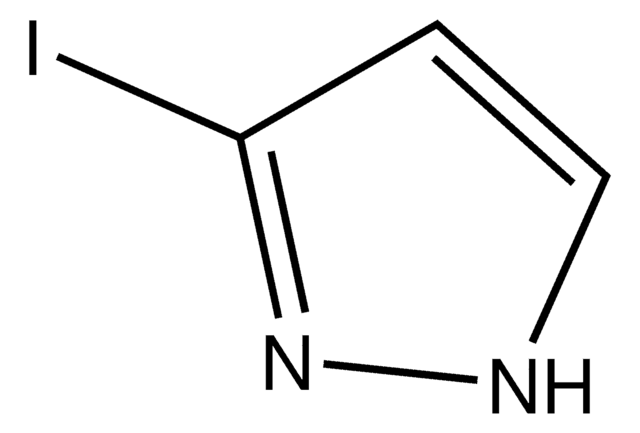
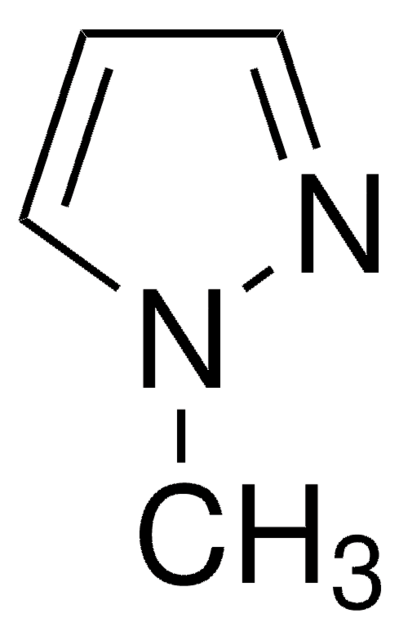
C3H3IN2
CAS Number:
Molecular Weight:
193.97
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
99%
form
solid
mp
108-110 °C (lit.)
SMILES string
Ic1cn[nH]c1
InChI
1S/C3H3IN2/c4-3-1-5-6-2-3/h1-2H,(H,5,6)
InChI key
LLNQWPTUJJYTTE-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
4-Iodopyrazole is a valuable intermediate for the synthesis of biologically active compounds. It undergoes iodination in the presence of iodine and ammonium hydroxide to yield 3,4-di-iodo- and 3,4,5-tri-iodo-pyrazole.
Application
4-Iodopyrazole was used in an indium-mediated synthesis of heterobiaryls.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral - Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Metin Zora et al.
The Journal of organic chemistry, 76(16), 6726-6742 (2011-07-12)
Electrophilic cyclizations of α,β-alkynic hydrazones by molecular iodine were investigated for the synthesis of 4-iodopyrazoles. α,β-Alkynic hydrazones were readily prepared by the reactions of hydrazines with propargyl aldehydes and ketones. When treated with molecular iodine in the presence of sodium
A Kojo et al.
Biochemical pharmacology, 42(9), 1751-1759 (1991-10-09)
Pyrazole and several of its derivatives increase the hepatic microsomal coumarin 7-hydroxylase to a variable extent. The strongest inducers are pyrazole itself and those derivatives which have a hydroxy group or a halogen at the 4-position of the molecule. The
Green iodination of pyrazoles with iodine/hydrogen peroxide in water.
Kim MM, et al.
Tetrahedron Letters, 49(25), 4026-4028 (2008)
Enrique Font-Sanchis et al.
The Journal of organic chemistry, 72(9), 3589-3591 (2007-04-05)
The palladium-mediated coupling reaction between triorganoindium reagents and organic electrophiles is extended to the synthesis of heteroaromatic compounds. Both electron-rich and electron-poor heterocycles can act as the organic electrophile or as the organoindium derivative.
Some iodinated pyrazole derivatives.
D Giles et al.
Journal of the Chemical Society. Perkin transactions 1, 13, 1179-1184 (1966-01-01)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service