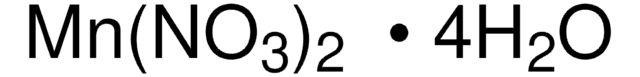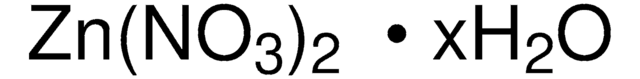203106
Cobalt(II) nitrate hexahydrate
99.999% trace metals basis
Synonym(s):
Cobaltous nitrate, Cobaltous nitrate hexahydrate, Nitric acid, cobalt(II) salt
About This Item
Recommended Products
Assay
99.999% trace metals basis
form
crystals and lumps
impurities
≤15.0 ppm Trace Metal Analysis
mp
55 °C (lit.)
application(s)
battery manufacturing
SMILES string
O.O.O.O.O.O.[Co++].[O-][N+]([O-])=O.[O-][N+]([O-])=O
InChI
1S/Co.2NO3.6H2O/c;2*2-1(3)4;;;;;;/h;;;6*1H2/q+2;2*-1;;;;;;
InChI key
QGUAJWGNOXCYJF-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
Application
- Used as a precursor to synthesize nano-Co3O4 via direct thermal decomposition method for electrochemical water oxidation applications
- Used as a precursor to synthesize single-crystalline Co3O4 powders with controllable morphology for lithium-ion battery applications. cobalt-containing nanomaterials, such as nano-Co3O4, with controlled size, shape, and morphology via sol-gel method.
- As a vital precursor to generate nickel-rich cathode materials (NMC, NCA) for lithium-ion batteries using co-precipitation process because of its simplicity, ease of scale-up, and ability to produce a homogeneous structure at the particle scale.
Cobalt(II) nitrate hexahydrate can be used as a:
- A starting material in the preparation of Co-based nanomaterials and Co complexes.
- A catalyst to synthesize 5-carboxanilide-dihydropyrimidinone derivatives via a three-component condensation reaction.
- A dopant to prepare LaCr1−xCoxO3 solid-solution ceramics for high temperature applications.
Features and Benefits
- High purity with trace metal analysis (=< 15 ppm) for 32 elements, suitable for batteries.
- High water solubility ideal for synthesizing composites for various applications.
- Low ppm levels of metal ions including Al, K, Na, Mg, Cu, Co, etc.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral - Aquatic Acute 1 - Aquatic Chronic 1 - Carc. 1B Inhalation - Eye Dam. 1 - Muta. 2 - Ox. Sol. 2 - Repr. 1B - Resp. Sens. 1 - Skin Sens. 1 - STOT RE 2 Inhalation
Target Organs
Lungs
Storage Class Code
5.1B - Oxidizing hazardous materials
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Articles
Advances in materials have often been led by the development of new synthetic methods that provide control over size, morphology and structure.
Lithium-ion batteries represent a group of electrochemical devices used for electricity storage and have attracted a lot of attention in the past two decades due to their portability, rechargeability and low cost.
Thermoelectric Performance of Perovskite-type Oxide Materials
Advances in materials have often been led by the development of new synthetic methods that provide control over size, morphology and structure. The preparation of materials in a scalable and continuous manner is critical when development moves beyond lab-scale quantities.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service