158461
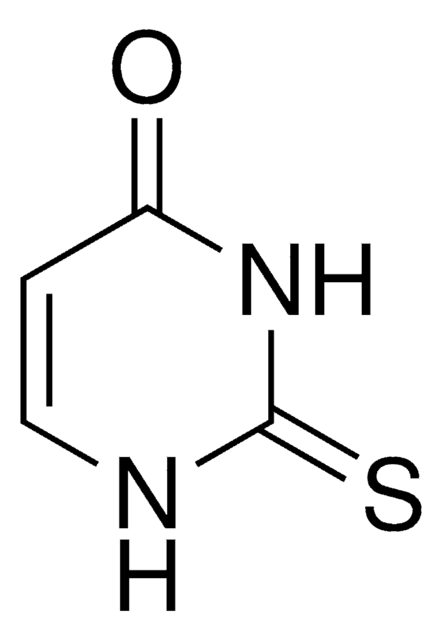
Dithiouracil
98%
Synonym(s):
2,4(1H,3H)-Pyrimidinedithione, 2,4-Dimercaptopyrimidine
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C4H4N2S2
CAS Number:
Molecular Weight:
144.22
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
Recommended Products
Quality Level
Assay
98%
mp
279-281 °C (dec.) (lit.)
SMILES string
S=C1NC=CC(=S)N1
InChI
1S/C4H4N2S2/c7-3-1-2-5-4(8)6-3/h1-2H,(H2,5,6,7,8)
InChI key
ZEQIWKHCJWRNTH-UHFFFAOYSA-N
General description
Dithiouracil is a potential anticancer drug and its redox mechanism and electronic absorption behavior has been investigated in a wide pH range by UV-Vis spectroscopy, cyclic voltammetry and differential pulse voltammetry.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Application of 2-thiouracil and 2,4-dithiouracil for the determination of metal ions. Part I. Spectrophotometric determination of copper and silver.
G Misztal et al.
Annales Universitatis Mariae Curie-Sklodowska. Sectio D: Medicina, 38, 135-141 (1983-01-01)
Afzal Shah et al.
Journal of photochemistry and photobiology. B, Biology, 117, 269-277 (2012-11-06)
The redox mechanism and electronic absorption behavior of a commercial anticancer drug, 5-fluorouracil (5-FU) and two potential anticancer drugs, 2-thiouracil (2-TU) and dithiouracil (DTU) have been investigated in a wide pH range by UV-Vis spectroscopy, cyclic voltammetry and differential pulse
Marvin Pollum et al.
ChemMedChem, 13(10), 1044-1050 (2018-03-14)
Sulfur-substituted nucleobases (i.e., thiobases) are a prospective class of compounds for clinical and cosmetic topical phototherapies. Recent investigations of several thiobases have revealed the ultrafast and efficient population of reactive triplet states upon ultraviolet-A (UVA) irradiation and the subsequent generation
Wen-Jwu Wang et al.
Organic & biomolecular chemistry, 3(16), 3054-3058 (2005-09-28)
2,4-dithiouracil (DTU) forms in the crystals the H-bonded monohydrates of a 1:1:1 ratio with 18-crown-6 (18C6) 1, cis,syn,cis-isomer of dicyclohexano-18-crown-6 (DCH6A) 2, and benzo-18-crown-6 (B18C6) 3, while the anhydrous adduct with cis,anti,cis-isomer of dicyclohexano-18-crown-6 (DCH6B) 4 is of a 2:1
L Lapinski et al.
Spectrochimica acta. Part A, Molecular and biomolecular spectroscopy, 54A(5), 685-693 (1998-07-29)
2,4-Pyrimidinedithiol (the dithiol form of 2,4-dithiouracil) was generated by UV (lambda > 335 nm) irradiation of the dithione form of 2,4-dithiouracil isolated in low-temperature argon or nitrogen matrices. The IR and UV spectra of the photoproduct are reported. The dithiol
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service