156507
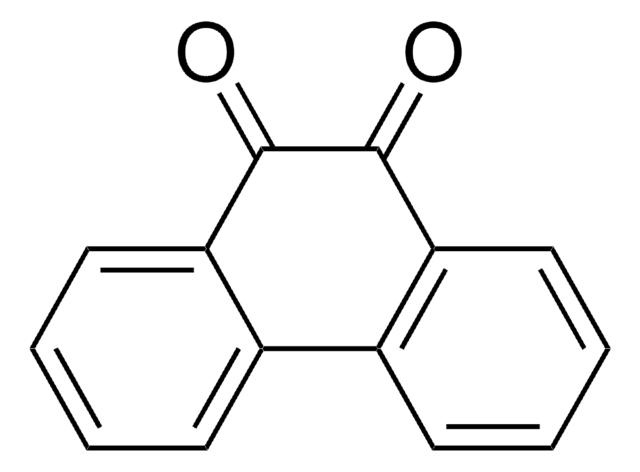
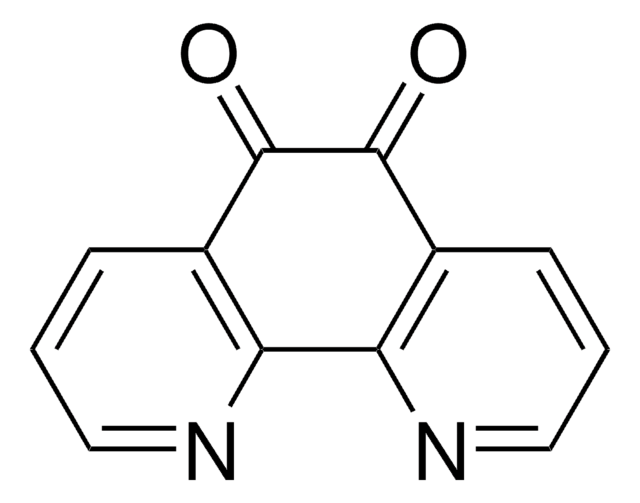
9,10-Phenanthrenequinone
≥99%
Synonym(s):
9,10-Phenanthrenedione
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Empirical Formula (Hill Notation):
C14H8O2
CAS Number:
Molecular Weight:
208.21
Beilstein:
608838
EC Number:
MDL number:
UNSPSC Code:
12352103
PubChem Substance ID:
NACRES:
NA.23
Recommended Products
Quality Level
Assay
≥99%
impurities
<0.1% anthraquinone
mp
209-212 °C (lit.)
λmax
420 nm
SMILES string
O=C1C(=O)c2ccccc2-c3ccccc13
InChI
1S/C14H8O2/c15-13-11-7-3-1-5-9(11)10-6-2-4-8-12(10)14(13)16/h1-8H
InChI key
YYVYAPXYZVYDHN-UHFFFAOYSA-N
Gene Information
human ... PTPN1(5770) , PTPRC(5788)
Looking for similar products? Visit Product Comparison Guide
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Aquatic Acute 1 - Eye Irrit. 2
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
473.0 °F
Flash Point(C)
245 °C
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
9, 10-Phenanthrenequinone binary complexes of iron, cobalt, and nickel
Floriani, C., R. Henzi, and F. Calderazzo
J. Chem. Soc., Dalton Trans., 23, 2640-2642 (1972)
Molecular Properties of 9, 10-Phenanthrenequinone and Benzil
Muddasir, H., Lu, P., Gu, C., Wang, Z. M., Yang, S. M., Yang, B., & Ma, Y. G.
Chemical Research in Chinese Universities, 25(6), 950-956 (2009)
Electronic structure and band alignment of 9, 10-phenanthrenequinone passivated silicon surfaces
Avasthi, Sushobhan, et al.
Surface Science, 605(13), 1308-1312 (2011)
Petr Milko et al.
Inorganic chemistry, 48(24), 11734-11742 (2009-11-26)
With the use of the model complexes [(PQ)FeCl(CH(3)O)](+), [(phen)FeCl(CH(3)O)](+), and [(PQ)(phen)FeCl(CH(3)O)](+), where PQ is 9,10-phenanthraquinone and phen is 1,10-phenanthroline, the reactivity of phenanthraquinone in complexes with iron(III) is investigated. It is shown that 9,10-phenanthraquinone takes part in redox processes occurring
Michael C Byrns et al.
Biochemical pharmacology, 75(2), 484-493 (2007-10-24)
Aldo-keto reductase (AKR) 1C3 (type 2 3alpha-HSD, type 5 17beta-HSD, and prostaglandin F synthase) regulates ligand access to steroid hormone and prostaglandin receptors and may stimulate proliferation of prostate and breast cancer cells. NSAIDs are known inhibitors of AKR1C enzymes.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service