112143
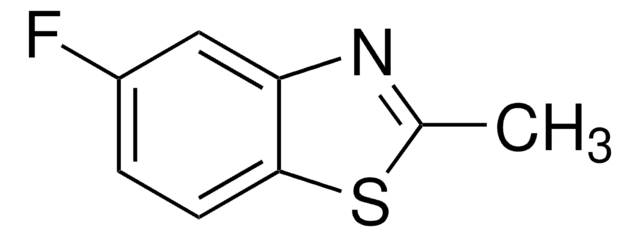
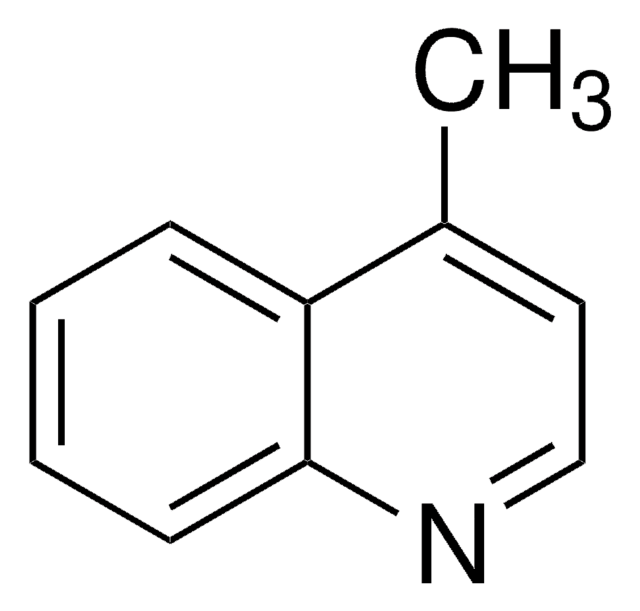
2-Methylbenzothiazole
99%
Synonym(s):
2-Methyl-1,3-benzothiazole, 2-Methylbenzo[d]thiazole
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Empirical Formula (Hill Notation):
C8H7NS
CAS Number:
Molecular Weight:
149.21
Beilstein:
112427
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
99%
refractive index
n20/D 1.617 (lit.)
bp
238 °C (lit.)
mp
11-14 °C (lit.)
density
1.173 g/mL at 25 °C (lit.)
SMILES string
Cc1nc2ccccc2s1
InChI
1S/C8H7NS/c1-6-9-7-4-2-3-5-8(7)10-6/h2-5H,1H3
InChI key
DXYYSGDWQCSKKO-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
2-Methylbenzothiazole can be synthesized by the Cu-catalyzed, base-free C-S coupling using conventional and microwave heating methods.
Application
Reagent employed in the synthesis of polycarbocyanine and thiacyanine dyes, as well as (arylfuryl)benzothiazoles.
Storage Class Code
10 - Combustible liquids
WGK
WGK 3
Flash Point(F)
215.6 °F - closed cup
Flash Point(C)
102 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Zh. Org. Khim., 28, 2159-2159 (1992)
Monatshefte fur Chemie / Chemical Monthly, 125, 209-209 (1994)
Chem. Abstr., 120, 194030z-194030z (1994)
Youji Huaxue, 14, 59-59 (1994)
Silvia M Soria-Castro et al.
Beilstein journal of organic chemistry, 9, 467-475 (2013-03-19)
S-aryl thioacetates can be prepared by reaction of inexpensive potassium thioacetate with both electron-rich and electron-poor aryl iodides under a base-free copper/ligand catalytic system. CuI as copper source affords S-aryl thioacetates in good to excellent yields, by using 1,10-phenanthroline as
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service