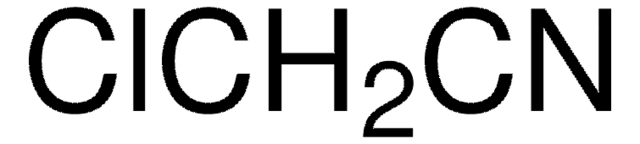109231
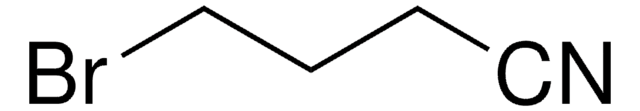
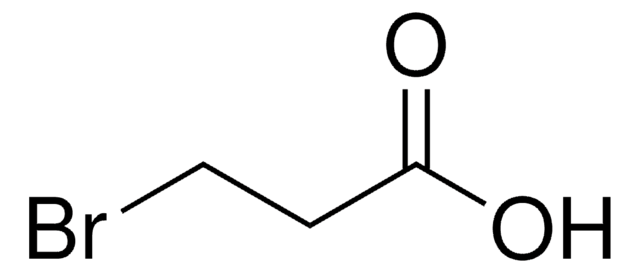
3-Bromopropionitrile
≥98.0% (GC)
Synonym(s):
3-Bromopropanenitrile
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
BrCH2CH2CN
CAS Number:
Molecular Weight:
133.97
Beilstein:
1738565
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
≥98.0% (GC)
refractive index
n20/D 1.481 (lit.)
bp
76-78 °C/10 mmHg (lit.)
density
1.615 g/mL at 25 °C (lit.)
functional group
bromo
nitrile
SMILES string
BrCCC#N
InChI
1S/C3H4BrN/c4-2-1-3-5/h1-2H2
InChI key
CQZIEDXCLQOOEH-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
Application
3-Bromopropionitrile was used as the initiator in the atom transfer radical polymerization of acrylonitrile. 3-Bromopropionitrile was used in coupling reactions of a number of aromatic and heteroaromatic phenols with alkyl, acyl or benzoyl halides in acetonitrile with cesium fluoride-Celite.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 2 Oral
Storage Class Code
6.1A - Combustible acute toxic Cat. 1 and 2 / very toxic hazardous materials
WGK
WGK 3
Flash Point(F)
206.6 °F - closed cup
Flash Point(C)
97 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Preparation of polyacrylonitrile with improved isotacticity and low polydispersity.
Jiang J, et al.
Journal of Applied Polymer Science, 116(5), 2610-2616 (2010)
Cesium fluoride-Celite: a solid base for efficient syntheses of aromatic esters and ethers.
Shah STA, et al.
Tetrahedron, 61(27), 6652-6656 (2005)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service