104558
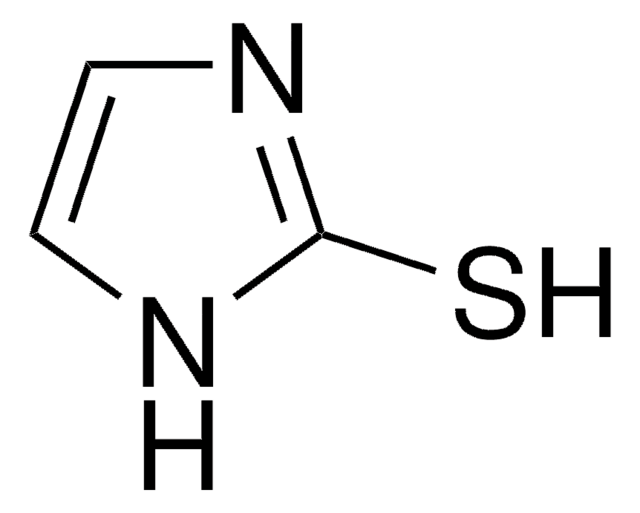
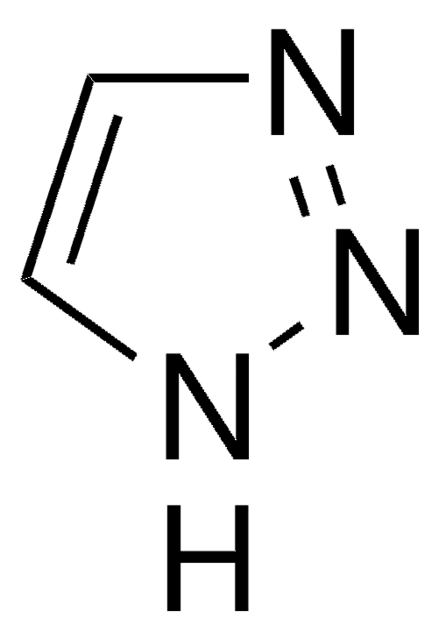
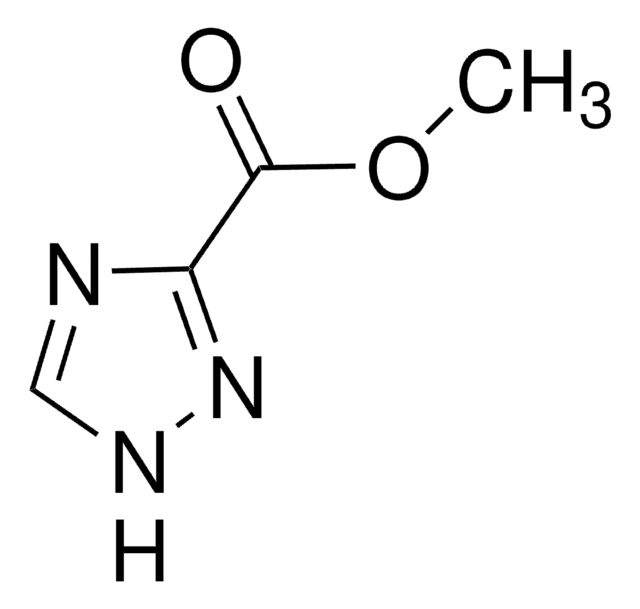
1H-1,2,4-Triazole-3-thiol
97%
Synonym(s):
3-Mercapto-1,2,4-triazole
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C2H3N3S
CAS Number:
Molecular Weight:
101.13
Beilstein:
107731
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
97%
mp
221-224 °C (lit.)
solubility
H2O: soluble 50 g/L
SMILES string
Sc1nc[nH]n1
InChI
1S/C2H3N3S/c6-2-3-1-4-5-2/h1H,(H2,3,4,5,6)
InChI key
AFBBKYQYNPNMAT-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
1H-1,2,4-Triazole-3-thiol is a mercapto-substituted 1,2,4-triazole ligand and exhibits tautomerism in solution. 1H-1,2,4-Triazole-3-thiol forms novel luminescent polymers with cadmium(II) salts. 1H-1,2,4-Triazole-3-thiol undergoes regioselective S-alkylation to form a series of S-substituted derivatives.
Application
1H-1,2,4-Triazole-3-thiol was used in a study to design a surface enhanced Raman scattering based probe for fast and accurate detection of DNA markers.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral - Eye Irrit. 2
Storage Class Code
11 - Combustible Solids
WGK
WGK 1
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Anion-induced coordination versatility of 1H-1, 2, 4-triazole-3-thiol (HtrzSH) affording a new hybrid system of cadmium (II) polymers: synthesis, structure, and luminescent properties.
Zhang RB, et al.
Crystal Growth & Design, 8(7), 2562-2573 (2008)
Synthesis of novel 1, 3-substituted 1H-[1, 2, 4]-triazole-3-thiol derivatives.
Eliazyan KA, et al.
Heteroatom Chem., 20(7), 405-410 (2009)
Lan Sun et al.
Analytical chemistry, 79(11), 3981-3988 (2007-05-01)
To provide rapid and accurate detection of DNA markers in a straightforward, inexpensive, and multiplex format, an alternative surface-enhanced Raman scattering based probe was designed and fabricated to covalently attach both DNA probing sequence and nonfluorescent Raman tags to the
Effect of some new antibilharzial drugs on oxygen consumption of mouse diaphragm and liver homogenates in experimental bilharziasis.
S M Amin et al.
Journal of the Egyptian Society of Parasitology, 12(1), 107-114 (1982-06-01)
A histochemical study of muscles of mice infected with Trichinella spiralis.
H N Awadalla et al.
Journal of the Egyptian Society of Parasitology, 12(1), 33-39 (1982-06-01)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service