CRM04291
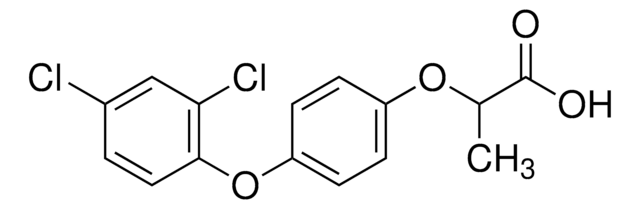
Diclofop-methyl
certified reference material, TraceCERT®, Manufactured by: Sigma-Aldrich Production GmbH, Switzerland
About This Item
Recommended Products
grade
certified reference material
TraceCERT®
Quality Level
product line
TraceCERT®
shelf life
limited shelf life, expiry date on the label
manufacturer/tradename
Manufactured by: Sigma-Aldrich Production GmbH, Switzerland
SMILES string
COC(=O)C(C)Oc1ccc(Oc2ccc(Cl)cc2Cl)cc1
InChI
1S/C16H14Cl2O4/c1-10(16(19)20-2)21-12-4-6-13(7-5-12)22-15-8-3-11(17)9-14(15)18/h3-10H,1-2H3
InChI key
BACHBFVBHLGWSL-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
Certified content by quantitative NMR incl. uncertainty and expiry date are given on the certificate.
Download your certificate at: http://www.sigma-aldrich.com
Diclofop-methyl is a post-emergence chiral herbicide that belongs to the class of aryloxyphenoxy propanoate (AOPP) compounds. It prevents the synthesis of fatty acids by inhibiting the activity of acetyl CoA carboxylase (ACCase). Diclofop-methyl is a selective and systemic herbicide used against wild oats, wild millets, and other annual grass weeds in broad-leaf crops, wheat, barley, maize, sorghum, oats, sugar cane, rice, dicotyledonous vegetables, and cotton. It causes an immediate inhibitory effect on the growth of the shoot, intercalary, and root meristems of the plant. It quickly hydrolyzes to give diclofop in plants, water, and soil.
Diclofop-methyl was included on 1st June 2011 in Annex I of Directive 91/414/EEC by the European Commission Directive 2011/45/EU. Diclofop-methyl is approved for use in the European Union under EC Regulation No 1107/2009, as per the Commission Implementing Regulation (EU) No 540/2011, however it is a candidate for substitution.
Application
The diclofop-methyl CRM may find its use as described below:
- To study the enantioselective degradation of diclofop-methyl and diclofop in two soil samples under aerobic and anaerobic conditions using high-performance liquid chromatography (HPLC)
- Simultaneous extraction and determination of enantiomers of diclofop-methyl and diclofop in cole using high-performance liquid chromatography-chiral stationary phase (HPLC-CS)
- Development of a multi-residue method for simultaneous estimation of 16 pesticides in water samples using direct immersion SPME followed by GC-MS analysis
- Simultaneous extraction and determination of aryloxyphenoxy-propionate herbicides from water samples by dispersive magnetic solid-phase extraction (d-MSPE) with high-performance liquid chromatography-diode array detector (HPLC-DAD) and ultra-high pressure liquid chromatography -triple quadrupole mass spectrometer (UHPLC-MS/MS)
- Evaluating the capacity of silica-bonded deoxycholic acid as a stationary phase having a 3,5-dinitrophenylcarbamoyl and a calix[4]arene substituent for enantiomeric separations
Recommended products
Legal Information
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral - Aquatic Acute 1 - Aquatic Chronic 1 - Skin Sens. 1
Storage Class Code
11 - Combustible Solids
WGK
WGK 2
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Choose from one of the most recent versions:
Certificates of Analysis (COA)
Don't see the Right Version?
If you require a particular version, you can look up a specific certificate by the Lot or Batch number.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service