P2645
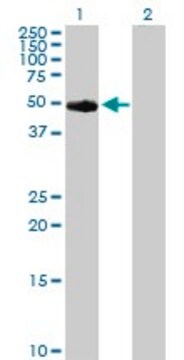
Protein Kinase A Catalytic Subunit from bovine heart
≥9 units/μg protein (cyclic-AMP is not required for this activity), lyophilized (white powder to sticky mass to hard pellet)
Synonym(s):
PKA
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
MDL number:
UNSPSC Code:
12352204
eCl@ss:
32160410
NACRES:
NA.32
Recommended Products
form
lyophilized (white powder to sticky mass to hard pellet)
Quality Level
specific activity
≥9 units/μg protein (cyclic-AMP is not required for this activity)
mol wt
40,862 Da
storage temp.
−20°C
General description
Protein Kinase A enzyme is composed of two subunits- catalytic and regulatory. The catalytic subunit exists as a monomer in the presence of cAMP and has a molecular weight of 40,862 Da.
Application
Protein Kinase A (PKA) Catalytic Subunit from bovine heart has been used-
- to study PKA-mediated inhibition of IRK1 (inwardly rectifying K+) channels
- in in Vitro PKA pPhosphorylation assay
- in Vitro affinity binding assays
- to study effects of PKA on inspiratory drive currents in functionally active motorneurons
Biochem/physiol Actions
Protein Kinase A (PKA) controls the transduction of Hedgehog signaling and participates in proliferation and fate specification. It phosphorylates several neurotransmitter receptors, transcription factors and constituents of various intracellular signaling pathways.
Protein Kinase A catalyzes the transfer of terminal phosphate from ATP to threonine or serine residues present on various proteins. This protein is inactive in the absence of cAMP, where the catalytic and regulatory subunits are bound together. The regulatory subunit, in the presence of cAMP, binds to cAMP and releases the catalytic subunit.
Packaging
Package size based on phosphorylating units
Unit Definition
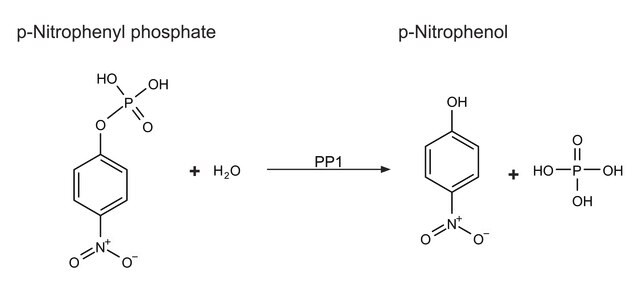
Phosphorylating Activity: One unit will transfer 1.0 picomole phosphate from ATP to hydrolyzed and partially dephosphorylated casein per minute at pH 6.5 at 30°C, determined by measuring the production of ADP.
Physical form
Lyophilized powder with sucrose and phosphate buffer salts as stabilizer.
Preparation Note
Prepared from protein kinase A (P 5511)
Disclaimer
Please note that the pack size has been changed to align with the unit definition, while the number of phosphorylating units remain the same as before.
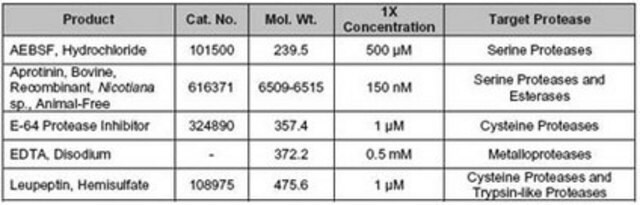
inhibitor
Product No.
Description
Pricing
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Cyclic nucleotides in the nervous system
Basic Neurochemistry, 423-441 (2012)
F T Hartl et al.
The Journal of biological chemistry, 258(6), 3950-3955 (1983-03-25)
The physical and chemical properties of purified catalytic and regulatory subunits of type II cAMP-dependent protein kinase from bovine brain, skeletal muscle, and cardiac muscle were compared. The catalytic subunits from all three sources were identical with respect to molecular
Ken Tougane et al.
Plant physiology, 152(3), 1529-1543 (2010-01-26)
Abscisic acid (ABA) is postulated to be a ubiquitous hormone that plays a central role in seed development and responses to environmental stresses of vascular plants. However, in liverworts (Marchantiophyta), which represent the oldest extant lineage of land plants, the
Christopher M Bocchiaro et al.
The Journal of neuroscience : the official journal of the Society for Neuroscience, 23(4), 1099-1103 (2003-02-25)
Plasticity underlying adaptive, long-term changes in breathing behavior is hypothesized to be attributable to the modulation of respiratory motoneurons by intracellular second-messenger cascades. In quiescent preparations, protein kinases, including cAMP-dependent protein kinase A (PKA), potentiate glutamatergic inputs. However, the dynamic
E Wischmeyer et al.
Proceedings of the National Academy of Sciences of the United States of America, 93(12), 5819-5823 (1996-06-11)
Strongly rectifying IRK-type inwardly rectifying K+ channels are involved in the control of neuronal excitability in the mammalian brain. Whole-cell patch-clamp experiments show that cloned rat IRK1 (Kir 2.1) channels, when heterologously expressed in mammalian COS-7 cells, are inhibited following
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service