M5255
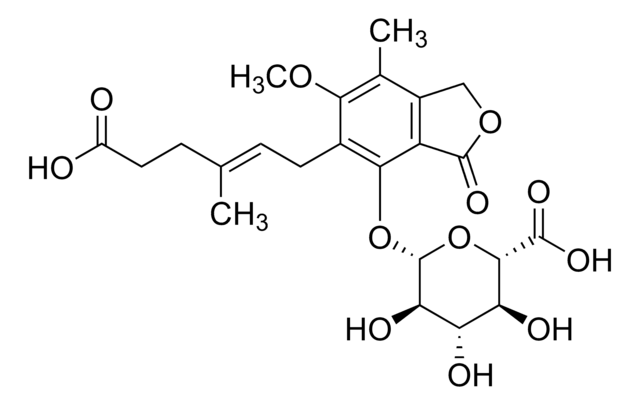
Mycophenolic acid
from Penicillium brevicompactum, ≥98% (HPLC), powder, IMP dehydrogenase inhibitor
Synonym(s):
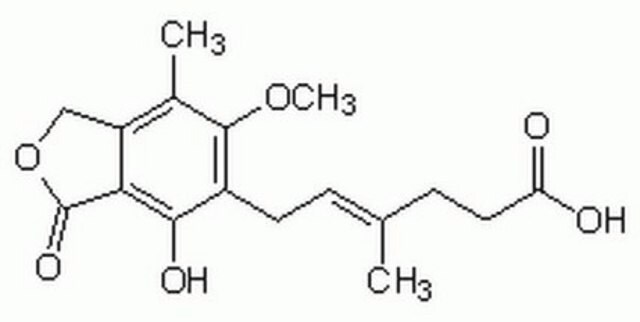
6-(1,3-Dihydro-7-hydroxy-5-methoxy-4-methyl-1-oxoisobenzofuran-6-yl)-4-methyl-4-hexanoic acid, 6-(4-Hydroxy-6-methoxy-7-methyl-3-oxo-5-phthalanyl)-4-methyl-4-hexenoic acid, NSC 129185
About This Item
Recommended Products
Product Name
Mycophenolic acid, ≥98%
biological source
Penicillium brevicompactum
Quality Level
Assay
≥98%
color
white to yellow-white
mp
<143.0 °C
solubility
methanol: 49.00-51.00 mg/mL, clear, colorless to faintly yellow
Mode of action
enzyme | inhibits
originator
Novartis
storage temp.
2-8°C
SMILES string
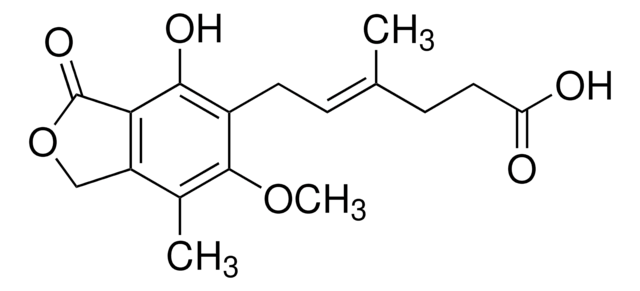
COc1c(C)c2COC(=O)c2c(O)c1C\C=C(/C)CCC(O)=O
InChI
1S/C17H20O6/c1-9(5-7-13(18)19)4-6-11-15(20)14-12(8-23-17(14)21)10(2)16(11)22-3/h4,20H,5-8H2,1-3H3,(H,18,19)/b9-4+
InChI key
HPNSFSBZBAHARI-RUDMXATFSA-N
Gene Information
human ... IMPDH1(3614) , IMPDH2(3615)
Looking for similar products? Visit Product Comparison Guide
Application
Biochem/physiol Actions
Features and Benefits
Caution
Preparation Note
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral - Aquatic Acute 1 - Aquatic Chronic 1 - Muta. 2 - Repr. 1B - STOT RE 1 Oral
Target Organs
Immune system
Storage Class Code
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Articles
Neoplastic cells are highly dependent on the de novo synthesis of nucleotides to maintain sufficient pools to support DNA replication and the production of RNA.
Related Content
We offer agonists, antagonists, modulators and other bioactive small molecules for immune system signaling target identification and validation, as well as a variety of antibiotics, antivirals, and antifungals.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service