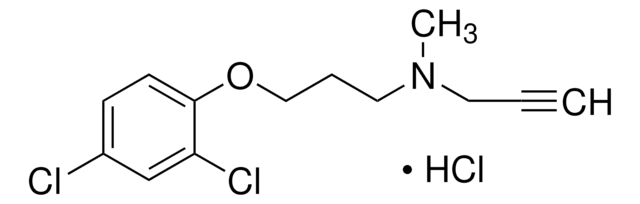
K3250
Kynuramine dihydrobromide
monoamine oxidase substrate, fluorogenic, ≥97% (TLC), crystalline
Synonym(s):
3-(2-Aminophenyl)-3-oxopropanamine
Sign Into View Organizational & Contract Pricing
All Photos(4)
About This Item
Empirical Formula (Hill Notation):
C9H12N2O · 2HBr
CAS Number:
Molecular Weight:
326.03
MDL number:
UNSPSC Code:
12352204
PubChem Substance ID:
NACRES:
NA.32
Recommended Products
Product Name
Kynuramine dihydrobromide, crystalline
Assay
≥97% (TLC)
Quality Level
form
crystalline
solubility
water: 50 mg/mL, clear to slightly hazy
storage temp.
−20°C
SMILES string
Br.NCCC(=O)c1ccccc1N
InChI
1S/C9H12N2O.BrH/c10-6-5-9(12)7-3-1-2-4-8(7)11;/h1-4H,5-6,10-11H2;1H
InChI key
YUPVVZSYBUIDQR-UHFFFAOYSA-N
Application
Kynuramine dihydrobromide has been used as a substrate for monoamine oxidase.
Biochem/physiol Actions
Kynuramine dihydrobromide is a substrate for the enzyme monoamine oxidase. The end product is the formation of fluorescence detectable 4-hydroxyquinoline (4-HOQ) measured in a fluorescence spectrophotometer at 380 nm.
Substrates
Substrate for monoamine oxidase
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Evidence for the involvement of the noradrenergic system, dopaminergic and imidazoline receptors in the antidepressant-like effect of tramadol in mice
Jesse CR, et al.
Pharmacology, Biochemistry, and Behavior, 95(3), 344-350 (2010)
Zhanna V Chirkova et al.
Drug development research, 79(2), 81-93 (2018-03-24)
Hit, Lead & Candidate Discovery In recent studies, we have shown that pyrrolo[3,4-f]indole-5,7-dione and indole-5,6-dicarbonitrile derivatives act as good potency in vitro inhibitors of the monoamine oxidase (MAO) enzymes. To expand on these series and to further derive structure-activity relationships
Chronic dietary mercury exposure causes oxidative stress, brain lesions, and altered behaviour in Atlantic salmon (Salmo salar) parr
Berntssen MHG, et al.
Aquatic Toxicology (Amsterdam, Netherlands), 65(1), 55-72 (2003)
Magdalena S Nel et al.
Bioorganic chemistry, 69, 20-28 (2016-09-24)
In the present study a series of fifteen 2-heteroarylidene-1-indanone derivatives were synthesised and evaluated as inhibitors of recombinant human monoamine oxidase (MAO) A and B. These compounds are structurally related to series of heterocyclic chalcone derivatives which have previously been
Synergistic antidepressant-like effect of ferulic acid in combination with piperine: involvement of monoaminergic system
Li G, et al.
Metabolic Brain Disease, 30(6), 1505-1514 (2015)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service