G6637
β-Galactose Dehydrogenase from Pseudomonas fluorescens
recombinant, expressed in E. coli, ammonium sulfate suspension, ≥50 units/mg protein (biuret)
Synonym(s):
D-Galactose:NAD+ 1-oxidoreductase
Sign Into View Organizational & Contract Pricing
Select a Size
All Photos(1)
Select a Size
Change View
About This Item
Recommended Products
biological source
Pseudomonas fluorescens
Quality Level
recombinant
expressed in E. coli
Assay
0.5—2.0 mg protein/mL (biuret)
form
ammonium sulfate suspension
specific activity
≥50 units/mg protein (biuret)
color
white
suitability
suitable for enzyme test
application(s)
life science and biopharma
shipped in
wet ice
Looking for similar products? Visit Product Comparison Guide
Application
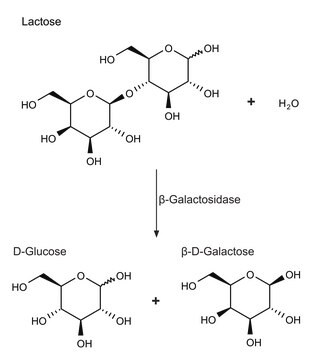
β-Galactose Dehydrogenase from Pseudomonas fluorescens has been used for competitive inhibition in lectin histochemistry. It has also been used to measure the hydrolysis activity of Haloferax alicantei β-galactosidase on different disaccharides.
Biochem/physiol Actions
β-galactose dehydrogenase catalyzes the oxidation of β-D-galactose to D-galactono-gammalactone.
Unit Definition
One unit will convert 1.0 μmole of D-galactose to D-galactonate per min at pH 8.6 at 25 °C.
Physical form
Suspension in 3.2 M (NH4)2SO4, pH approx. 6.0
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
H Pettersson et al.
Biochimica et biophysica acta, 1549(2), 155-160 (2001-11-03)
The mechanistic implications of the kinetic behaviour of a fusion protein of beta-galactosidase and galactose dehydrogenase have been analysed in view of predictions based on experimentally determined kinetic parameter values for the galactosidase and dehydrogenase activities of the protein. The
R D Hancock et al.
FEMS microbiology letters, 186(2), 245-250 (2000-05-10)
Saccharomyces cerevisiae cells incubated with D-glucose (D-Glc), D-galactose or D-mannose (D-Man) synthesised D-erythroascorbic acid (D-EAA) but not L-ascorbic acid (L-AA). Accumulation of D-EAA was observed in cells incubated with D-arabinose (D-Ara) whilst accumulation of L-AA occurred in cells incubated with
C F Mazitsos et al.
Journal of chromatography. A, 1029(1-2), 103-112 (2004-03-23)
Two chimaeric galactosyl-mimodye ligands were designed and applied to the purification of Pseudomonas fluorescens galactose dehydrogenase (GaDH). The chimaeric affinity ligands comprised a triazine ring on which were anchored: (i) an anthraquinone moiety that pseudomimics the adenine part of NAD+
Michael Sauer et al.
Applied and environmental microbiology, 70(10), 6086-6091 (2004-10-07)
Yeasts do not possess an endogenous biochemical pathway for the synthesis of vitamin C. However, incubated with l-galactose, L-galactono-1,4-lactone, or L-gulono-1,4-lactone intermediates from the plant or animal pathway leading to l-ascorbic acid, Saccharomyces cerevisiae and Zygosaccharomyces bailii cells accumulate the
Virapong Prachayasittikul et al.
International journal of biological sciences, 2(1), 10-16 (2006-04-06)
A chimeric bifunctional enzyme composing of galactose dehydrogenase (galDH; from Pseudomonas fluorescens) and lactate dehydrogenase (LDH; from Bacillus stearothermophilus) was successfully constructed. The chimeric galDH/LDH possessed dual characteristics of both galactose dehydrogenase and lactate dehydrogenase activities while exhibiting hexameric rearrangement
Articles
Instructions for working with enzymes supplied as ammonium sulfate suspensions
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service