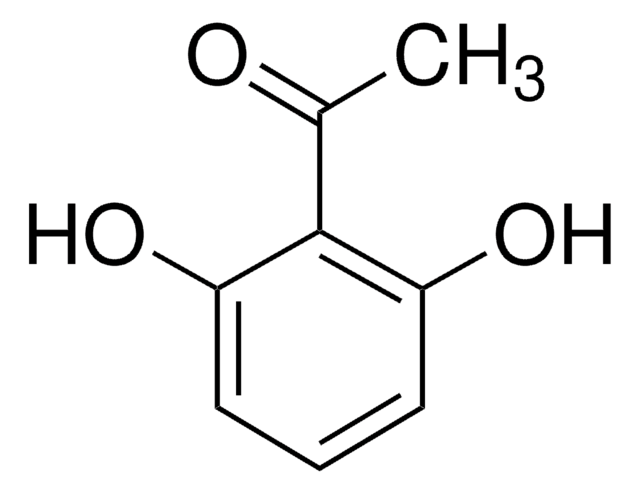
37442
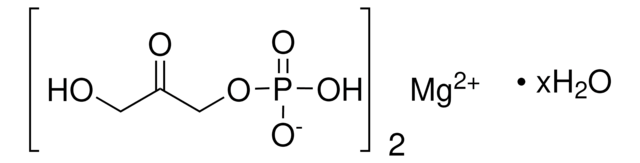
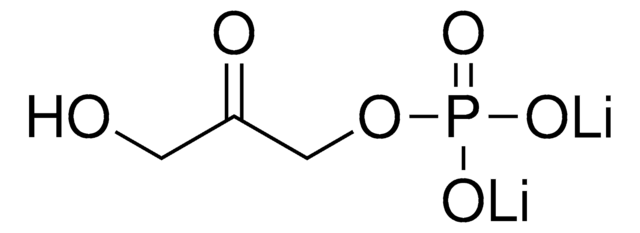
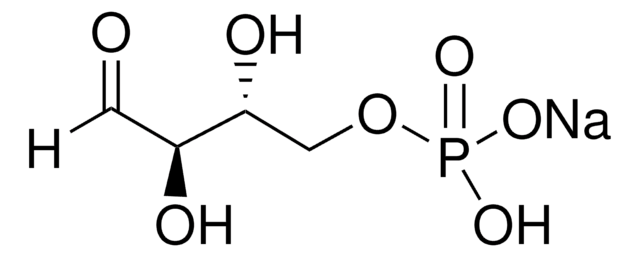
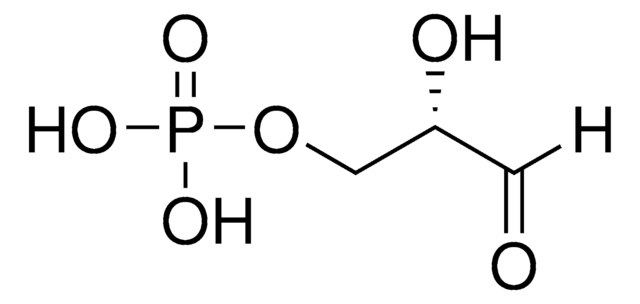
Dihydroxyacetone phosphate lithium salt
≥95.0% (TLC)
Synonym(s):
1-Hydroxy-3-(phosphonooxy)-2-propanone lithium salt, DHAP, Glycerone phosphate lithium salt
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C3H7O6P · xLi+
CAS Number:
Molecular Weight:
170.06 (free acid basis)
MDL number:
UNSPSC Code:
12352204
PubChem Substance ID:
NACRES:
NA.32
Recommended Products
Assay
≥95.0% (TLC)
form
powder
storage temp.
−20°C
SMILES string
OCC(COP(O)(O)=O)=O
InChI
1S/C3H7O6P/c4-1-3(5)2-9-10(6,7)8/h4H,1-2H2,(H2,6,7,8)
InChI key
GNGACRATGGDKBX-UHFFFAOYSA-N
Related Categories
Biochem/physiol Actions
Dihydroxyacetone phosphate (DHAP) is a metabolic intermediate involved in many pathways, including glycolysis, gluconeogenesis, glycerol metabolism, phosphatidic acid synthesis, fat metabolism, and the Calvin cycle.
Analysis Note
may contain up to 2-mol-equivalents water
replaced by
Product No.
Description
Pricing
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Jun Ogawa et al.
Bioscience, biotechnology, and biochemistry, 67(4), 933-936 (2003-06-06)
2-Deoxyribose 5-phosphate was produced from acetaldehyde and dihydroxyacetone phosphate via D-glyceraldehyde 3-phosphate by Klebsiella pneumoniae B-4-4 through deoxyriboaldolase- and triosephosphate isomerase-catalyzing reactions. Under the optimum conditions, 98.7 mM 2-deoxyribose 5-phosphate was produced from 200 mM acetaldehyde and 117 mM dihydroxyacetone
Parul Agarwal et al.
Plant cell reports, 38(10), 1235-1248 (2019-06-14)
Using, in silico, in vitro and in planta functional assays, we demonstrate that Ps3'OMT, an 3'-O methyl transferase is linked to papaverine biosynthesis in opium poppy. Papaverine, one of the benzylisoquinoline alkaloids (BIA) synthesized in the medicinally important plant, Papaver
Glycerolipid biosynthesis in peroxisomes via the acyl dihydroxyacetone phosphate pathway.
A K Hajra et al.
Annals of the New York Academy of Sciences, 386, 170-182 (1982-01-01)
John P Richard
ACS chemical biology, 3(10), 605-607 (2008-10-22)
Gluconeogenesis is blocked in a strain of Escherichia coli that is deficient in triosephosphate isomerase, but it was restored by the insertion of a plasmid coding for an L-glyceraldehyde 3-phosphate reductase (YghZ). This reductase provides a "bypass" that produces dihydroxyacetone
Giovanni Covaleda-Cortés et al.
Marine drugs, 17(9) (2019-09-01)
A very powerful proteinaceous inhibitor of metallocarboxypeptidases has been isolated from the marine snail Nerita versicolor and characterized in depth. The most abundant of four, very similar isoforms, NvCla, was taken as reference and N-terminally sequenced to obtain a 372-nucleotide
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service