19924
Pyroglutamate Aminopeptidase from Pyrococcus furiosus, recombinant from E. coli
7-13 mU (per vial)
Synonym(s):
Pyrase, Pyroglutamyl-Peptidase I, Pyrrolidone Carboxyl Peptidase
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Recommended Products
recombinant
expressed in E. coli
Quality Level
form
lyophilized
specific activity
7-13 mU (per vial)
mol wt
Mr ~28000
storage temp.
−20°C
Related Categories
Packaging
package of 0.01 Unit
Unit Definition
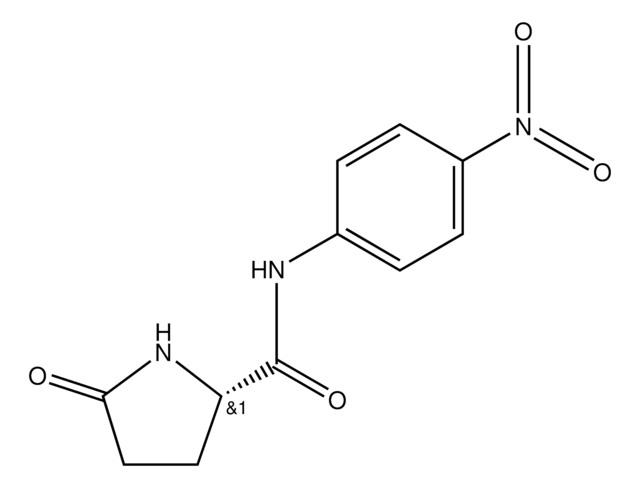
1 U corresponds to the amount of enzyme which hydrolyzes 1 μmol Pyroglutamate-p-nitroanilide per minute at pH 7.0 and 37 °C
Analysis Note
enzyme activity: the optimum temperature is 95-100 °C (the enzyme is stable up to 75 °C), the optimum pH is 6-9 (stable from pH 6-9). Inhibitors: PCMB, Hg+.
Other Notes
An enzyme that removes pyroglutamic acid (pGlu) from pGlu-peptide and proteins; employed in Edman degradation.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Resp. Sens. 1 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
J Mozdzanowski et al.
Analytical biochemistry, 260(2), 183-187 (1998-07-11)
For larger proteins, efficient deblocking prior to Edman sequencing is especially important to obtain quality, extended sequencing data which is limited by the stepwise accumulation of background from the random acid hydrolysis of the protein. Therefore, any portion that remains
Y Shimada et al.
Journal of biochemistry, 106(3), 383-388 (1989-09-01)
The cDNA clone of Geotrichum candidum (Geo.) lipase was isolated from the Geo. cDNA library by colony hybridization using 32P-labeled oligonucleotides corresponding to a partial amino acid sequence of this enzyme. The nucleotide sequence of the cDNA determined by the
Marie Schaeffer et al.
Molecular and biochemical parasitology, 150(2), 318-329 (2006-10-10)
Pyroglutamyl peptidases I (PPI) are cysteine peptidases of the clan CF, family C15, which hydrolyse N-terminal l-pyroglutamyl residues (l-pGlu). The l-pGlu modification is a post-transcriptional modification that confers relative aminopeptidase resistance and, in some cases, is essential to the modified
Johanna L Hellström et al.
Biochimica et biophysica acta, 1764(11), 1735-1740 (2006-10-20)
To enable Edman sequencing of pyroglutamylated immunoglobulins, enzymatic deblocking by pyroglutamate aminopeptidase is performed, often with variable yield and compromised solubility. Recently, enzymatic deblocking of immunoglobulins without denaturation was described. Although the conditions ensured efficient removal of pyroglutamyl residues, we
Noriko Higaki-Sato et al.
Journal of agricultural and food chemistry, 54(19), 6984-6988 (2006-09-14)
In order to determine pyroglutamic acid levels in plasma, we developed a method based on precolumn derivatization of the carboxyl group of pyroglutamic acid with 2-nitrophenylhydrazine. Eight-week-old male SD strain rats were administered 200 mg of an acidic peptide fraction
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service