11625
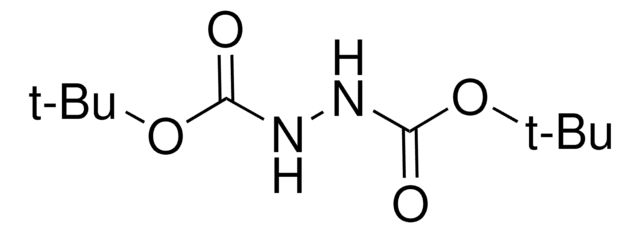
Di-tert-butyl azodicarboxylate
purum, ≥98.0% (GC)
Synonym(s):
Bis(1,1-dimethylethyl)azodicarboxylate, DBAD, Di-tert-butyl azodiformate, NSC 109889
About This Item
Recommended Products
grade
purum
Quality Level
Assay
≥98.0% (GC)
form
solid
mp
89-92 °C (lit.)
89-92 °C
functional group
azo
storage temp.
2-8°C
SMILES string
CC(C)(C)OC(=O)\N=N\C(=O)OC(C)(C)C
InChI
1S/C10H18N2O4/c1-9(2,3)15-7(13)11-12-8(14)16-10(4,5)6/h1-6H3/b12-11+
InChI key
QKSQWQOAUQFORH-VAWYXSNFSA-N
Looking for similar products? Visit Product Comparison Guide
General description
Application
- Preparation of hexapeptide key fragments via stereoselective selenocyclization/oxidative deselenylation or hydrazination/cyclization reactions
- Asymmetric Michael addition reactions
- Preparation of dipeptidyl peptidase IV dependent water-soluble prodrugs via Mitsunobu reaction
- Synthesis of pyrroloisoquinoline template via stereoselective N-acyliminium-mediated cyclization and enolate amination for synthesis of peptidomimetic compounds
- Barbier-type propargylation reactions
- Synthesis of bacterial peptide deformylase (PDF) inhibitor fumimycin
- Asymmetric amination of glycine Schiff bases
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
Choose from one of the most recent versions:
Certificates of Analysis (COA)
It looks like we've run into a problem, but you can still download Certificates of Analysis from our Documents section.
If you need assistance, please contact Customer Support.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service