PHR1428
HEPES
certified reference material,pharmaceutical secondary standard
Synonym(s):
HEPES, 4-(2-Hydroxyethyl)piperazine-1-ethanesulfonic acid, N-(2-Hydroxyethyl)piperazine-N′-(2-ethanesulfonic acid)
About This Item
product name
HEPES, Pharmaceutical Secondary Standard; Certified Reference Material
grade
certified reference material
pharmaceutical secondary standard
Quality Level
API family
hepes
CofA
current certificate can be downloaded
technique(s)
HPLC: suitable
gas chromatography (GC): suitable
pH
5.0-6.5 (25 °C, 238 g/L)
useful pH range
6.8-8.2
pKa (25 °C)
7.5
application(s)
pharmaceutical (small molecule)
format
neat
storage temp.
2-30°C
SMILES string
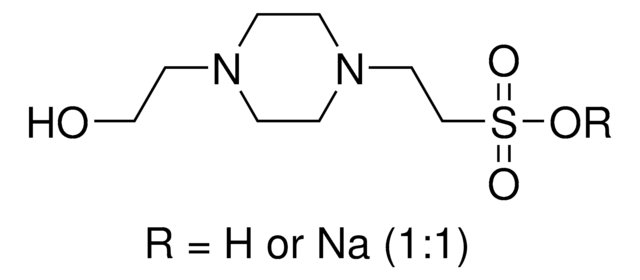
OCCN1CCN(CC1)CCS(O)(=O)=O
InChI
1S/C8H18N2O4S/c11-7-5-9-1-3-10(4-2-9)6-8-15(12,13)14/h11H,1-8H2,(H,12,13,14)
InChI key
JKMHFZQWWAIEOD-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
Pharmaceutical secondary standards for application in quality control, provide pharma laboratories and manufacturers with a convenient and cost-effective alternative to the preparation of in-house working standards.
Application
- HEPES has been used in a wide variety of applications, including tissue culture.
- It is used to buffer cell culture media in air.
- Finds its usage in invitro experiments on Mg.
- Recognized as one of Dr.Good′s recommended buffers, used in some cell culture media as a buffering agent and also in various biochemical reactions.
- Recently, in the production of radiopharmaceuticals, it is the buffer of choice for scientific labeling.
HEPES may also be used as:
- Component of homogenization buffer to homogenize adipose tissue for liquid chromatography/mass spectrometry and in HN buffer for dissolving viral stock titer after dot blot hybridization.
- Pharmaceutical reference standard for the determination of the analyte in bulk drug and pharmaceutical formulations by high performance liquid chromatography.
Analysis Note
Other Notes
Footnote
Storage Class Code
13 - Non Combustible Solids
WGK
WGK 1
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service