52650
Hexamethylene diisocyanate
purum, ≥98.0% (GC)
Synonym(s):
1,6-Diisocyanatohexane
About This Item
Recommended Products
grade
purum
Quality Level
Assay
≥98.0% (GC)
refractive index
n20/D 1.453
bp
82-85 °C/0.1 mmHg
density
1.047 g/mL at 20 °C (lit.)
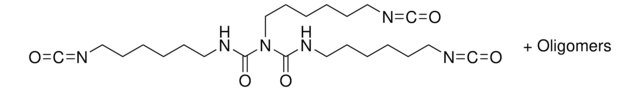
SMILES string
O=C=NCCCCCCN=C=O
InChI
1S/C8H12N2O2/c11-7-9-5-3-1-2-4-6-10-8-12/h1-6H2
InChI key
RRAMGCGOFNQTLD-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
Application
- A crosslinker to crosslink the polyurethane chains in the triblock copolymer gate dielectric, which is then deposited on the substrate to fabricate low-voltage organic thin-film transistors.
- A precursor in the preparation of electroactive shape memory polyurethane/graphene nanocomposites. These materials are usually used as actuators, sensors, artificial muscles, smart devices, and microswitches.
- A crosslinker in conjunction with Pluronic F127, a nonionic surfactant, to synthesize a poly(lactic acid) (PLA)-based hydrogel for biomedical applications.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 1 Inhalation - Acute Tox. 4 Oral - Eye Dam. 1 - Resp. Sens. 1 - Skin Corr. 1C - Skin Sens. 1 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
6.1A - Combustible acute toxic Cat. 1 and 2 / very toxic hazardous materials
WGK
WGK 1
Flash Point(F)
266.0 °F - Pensky-Martens closed cup
Flash Point(C)
130 °C - Pensky-Martens closed cup
Personal Protective Equipment
Regulatory Listings
Regulatory Listings are mainly provided for chemical products. Only limited information can be provided here for non-chemical products. No entry means none of the components are listed. It is the user’s obligation to ensure the safe and legal use of the product.
EU REACH Annex XVII (Restriction List)
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Protocols
Separation of Methyl isocyanate; Ethyl isocyanate, 98%; Propyl isocyanate, 99%; Phenyl isocyanate, for HPLC derivatization, ≥99.0% (GC); Hexamethylene diisocyanate, puriss., ≥99.0% (GC)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service