1.01116
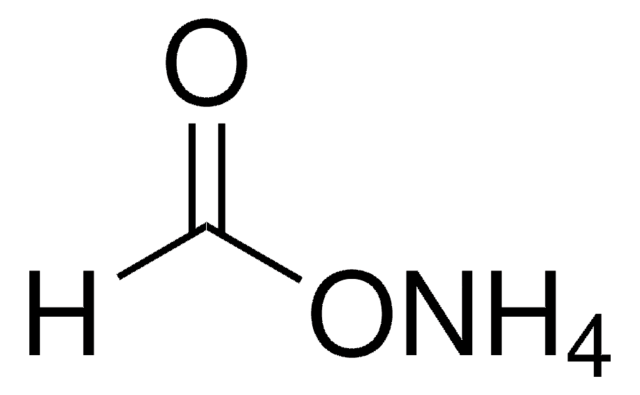
Ammonium acetate
for analysis EMSURE® ACS,Reag. Ph Eur
Synonym(s):
Ammonium acetate
About This Item
Recommended Products
grade
ACS reagent
Quality Level
Agency
reag. Ph. Eur.
product line
EMSURE®
Assay
≥98.0% (acidimetric)
form
solid
impurities
≤0.005% Insoluble matter
≤0.005% Substances reducing potassium permanganate (as formic acid)
≤2% Water
ign. residue
≤0.01% (as sulfate)
pH
6.7-7.3 (25 °C, 50 g/L in H2O)
mp
114 °C
density
1.17 g/cm3 at 20 °C
bulk density
410 kg/m3
anion traces
chloride (Cl-): ≤0.0005%
nitrate (NO3-): ≤0.001%
sulfate (SO42-): ≤0.001%
cation traces
Ca: ≤0.001%
Fe: ≤0.0002%
heavy metals (as Pb): ≤0.0002%
storage temp.
15-25°C
InChI
1S/C2H4O2.H3N/c1-2(3)4;/h1H3,(H,3,4);1H3
InChI key
USFZMSVCRYTOJT-UHFFFAOYSA-N
Related Categories
Application
- Ammonium Acetate in Buffer Preparation: A study highlighted the use of ammonium acetate in buffer solutions for molecular biology applications, emphasizing its role in maintaining pH stability and compatibility with various biochemical processes (Ahamed et al., 2024).
- High-Purity Ammonium Acetate for Mass Spectrometry: Another research employed high-purity ammonium acetate in developing a robust mass spectrometry method for the analysis of complex biological samples, demonstrating its effectiveness in improving detection sensitivity and accuracy (Mozayad et al., 2024).
- Material Science Applications: In the field of material science, ammonium acetate has been used as a precursor for the synthesis of novel materials with potential applications in energy storage and electronics, showcasing its versatility and functional importance (Hansen et al., 2024).
- Pharmaceutical Synthesis: Highlighting its role in pharmaceutical formulations, a study discussed the use of ammonium acetate as a catalyst in drug synthesis processes, contributing to more efficient and cleaner production methods (Zhao et al., 2024).
- Environmental Testing Reagent: Ammonium acetate is also pivotal in environmental science, particularly in the analysis of soil and water samples for pollution assessment, where it acts as an effective ion pairing agent to measure various pollutants (Kopeć et al., 2024).
Linkage
Analysis Note
Insoluble matter: ≤ 0.005 %
pH-value (5 %; water, 25 °C): 6.7 - 7.3
Chloride (Cl): ≤ 0.0005 %
Nitrate (NO₃): ≤ 0.001 %
Sulfate (SO₄): ≤ 0.001 %
Heavy metals (as Pb): ≤ 0.0002 %
Ca (Calcium): ≤ 0.001 %
Fe (Iron): ≤ 0.0002 %
Substances reducing potassium permanganate (as formic acid): ≤ 0.005 %
Residue on ignition (as sulfate): ≤ 0.01 %
Water: ≤ 2.0 %
Corresponds to ACS,Reag. Ph Eur
Legal Information
Storage Class Code
11 - Combustible Solids
WGK
WGK 1
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service