810600P
Avanti
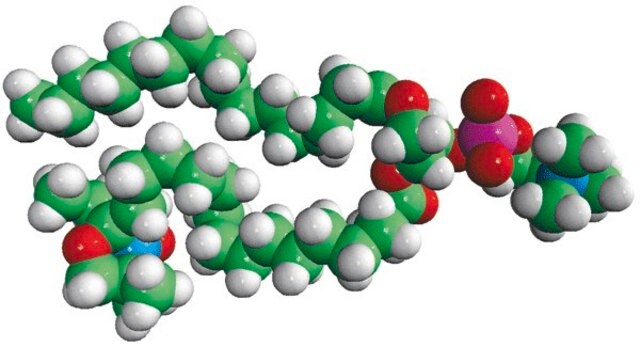
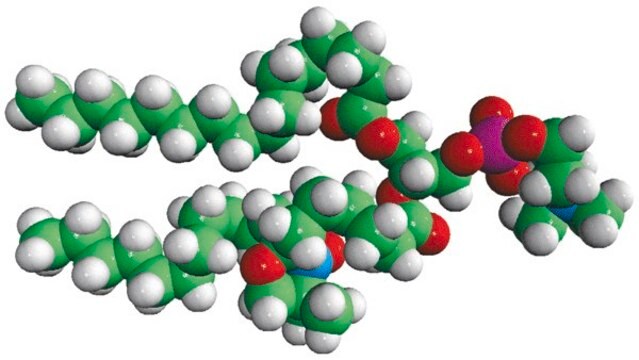
16:0-12 Doxyl PC
Avanti Research™ - A Croda Brand 810600P, powder
Synonym(s):
1-palmitoyl-2-stearoyl-(12-doxyl)-sn-glycero-3-phosphocholine
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C46H90N2O10P
CAS Number:
Molecular Weight:
862.19
UNSPSC Code:
41141825
NACRES:
NA.25
Recommended Products
Assay
>99% (TLC)
form
powder
packaging
pkg of 1 × 1 mg (810600P-1mg)
manufacturer/tradename
Avanti Research™ - A Croda Brand 810600P
lipid type
ESR probes
phospholipids
shipped in
dry ice
storage temp.
−20°C
General description
Avanti′s nitroxide spin product listing is a group of compounds designed to act as membrane probes. A variety of positions down the hydrophobic chain are labeled with the nitroxide functional groups to allow probing the membrane at various depths. These compounds have been synthesized from 1-palmitoyl-2-hydroxy-sn-glycerol-3-phosphocholine with the product being purified by column chromatography. Various n-doxyl phosphocholines have been recently used as biophysical tools to elucidate membrane trafficking with phosphatidylinositol transfer proteins and as fluorescent quenchers in lipid bilayer structural studies.
Phosphatidylcholine (PC) is a strong bilayer-forming lipid. It the most common phospholipid in mammalian membranes. It is also an important component of the mucosal layer of the colon. 1-palmitoyl-2-stearoyl-(12-doxyl)-sn-glycero-3-phosphocholine (12-NO-PC) is a nitroxide-labeled phospholipid.
Application
16:0-12 Doxyl PC is suitable for use:
- as a lipophilic collisional quencher to prepare liposomes used in lipophilic quenching experiments
- to prepare liposomes used in nitroxide quenching experiments to examine the location of each NBD (7-nitrobenz-2-oxa-1,3-diazole) probe in mutants
- as a component in POPC or 1:1 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine (POPC)/1-palmitoyl-2-oleoyl-sn-glycero-3-phospho-rac-1-glycerol (POPG) mixtures to prepare unlabelled large unilamellar liposomes
Biochem/physiol Actions
Phosphatidylcholine (PC) functions as a surfactant within the mucus to form a hydrophobic surface to inhibit bacterial penetrance. It is used to treat fat embolism. Phosphatidylcholine lowers the levels of cholesterol and triglycerides.
Packaging
5 mL Clear Glass Sealed Ampule (810600P-1mg)
Preparation Note
To prevent aggregation, prepare water-based solutions of 2 mM stock solutions of n-DOXYL PCs and store in plastic. Dilute stock solutions to 0.03- 0.1 mM solutions for EPR studies. For liposome preparations in fluorescent quenching measurements, dissolve the doxyl lipid in 150 μl absolute ethanol for a concentration of 40.3 mM. href="https://pubs.acs.org/doi/suppl/10.1021/ja804929m/suppl_file/ja804929m_si_001.pdf"target="_blank">Supplemental information
Legal Information
Avanti Research is a trademark of Avanti Polar Lipids, LLC
also commonly purchased with this product
Product No.
Description
Pricing
Storage Class Code
11 - Combustible Solids
Choose from one of the most recent versions:
Certificates of Analysis (COA)
Lot/Batch Number
Sorry, we don't have COAs for this product available online at this time.
If you need assistance, please contact Customer Support.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Lipid-specific binding of the calcium-dependent antibiotic daptomycin leads to changes in lipid polymorphism of model membranes
Jung D, et al.
Chemistry and Physics of Lipids, 154(2), 120-128 (2008)
The Membranes of Cells, 154(2), 120-128 (2016)
Integrative Medicine - E-Book, 154(2), 120-128 (2017)
Structural insights into the membrane-anchoring mechanism of a cholesterol-dependent cytolysin
Ramachandran R, et al.
Nature Structural and Molecular Biology, 9(11), 823-823 (2002)
The mechanism of membrane insertion for a cholesterol-dependent cytolysin: a novel paradigm for pore-forming toxins
Shatursky O, et al.
Cell, 99(3), 293-299 (1999)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service