ALD00106
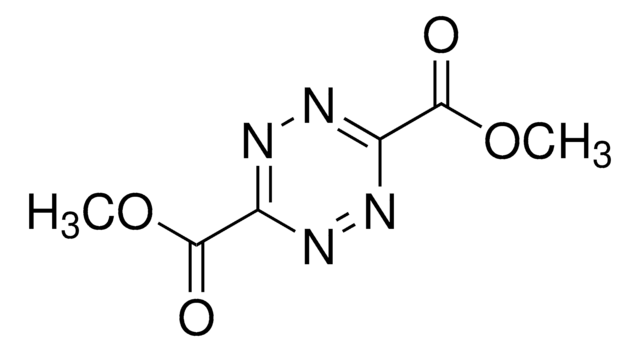
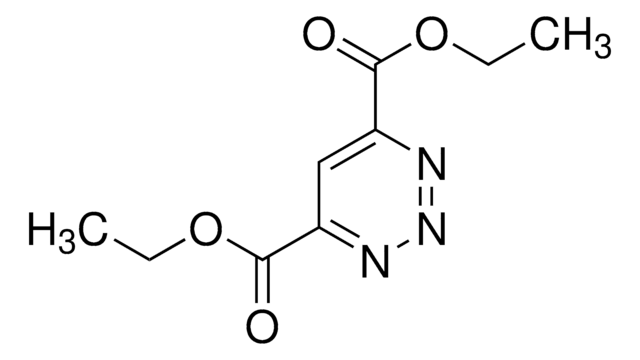
5-Methoxycarbonyl-1,2,3-triazine
Synonym(s):
1,2,3-Triazine-5-carboxylic acid, methyl ester
About This Item
Recommended Products
form
powder
storage temp.
−20°C
SMILES string
O=C(OC)C1=CN=NN=C1
InChI
1S/C5H5N3O2/c1-10-5(9)4-2-6-8-7-3-4/h2-3H,1H3
InChI key
DZONTPIFZFRSGP-UHFFFAOYSA-N
Application
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Choose from one of the most recent versions:
Certificates of Analysis (COA)
Don't see the Right Version?
If you require a particular version, you can look up a specific certificate by the Lot or Batch number.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Articles
The inverse electron demand Diels-Alder reactions of electron-deficient heterocycles are significant cycloaddition reactions for the total synthesis of natural products containing highly substituted and functionalized heteroaromatic ring systems.
Related Content
As the exploration of the properties of complex natural products becomes increasingly more sophisticated with the technological advances being made in their screening and evaluation and as structural details of their interaction with biological targets becomes more accessible, the importance and opportunities for providing unique solutions to complex biological problems has grown. The Boger Lab addresses these challenging problems by understanding the complex solutions and subtle design elements that nature has provided in the form of a natural product and work to extend the solution through rational design elements to provide more selective, more efficacious, or more potent agents designed specifically for the problem or target under investigation. The resulting efforts have reduced many difficult or intractable synthetic challenges to manageable problems providing an approach not only to the natural product but one capable of simple extrapolation to a series of structural analogs with improved selectivity and efficacy.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service