A79000
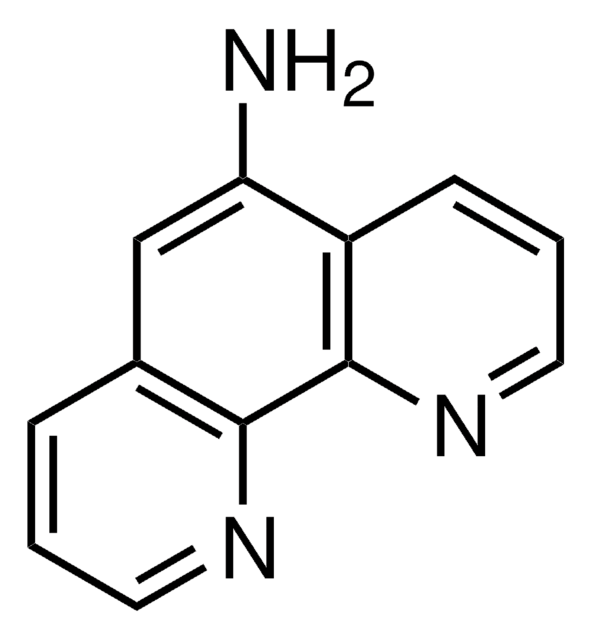
4-Aminoquinaldine
98%
Synonym(s):
2-Methyl-4-quinolinamine, 4-Amino-2-methylquinoline, 4-Quinaldinamine, NSC 60281
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C10H10N2
CAS Number:
Molecular Weight:
158.20
Beilstein:
118323
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
98%
form
powder
bp
333 °C (lit.)
mp
162-166 °C (lit.)
SMILES string
Cc1cc(N)c2ccccc2n1
InChI
1S/C10H10N2/c1-7-6-9(11)8-4-2-3-5-10(8)12-7/h2-6H,1H3,(H2,11,12)
InChI key
COCFIBRMFPWUDW-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Ishani Deb et al.
Bioorganic & medicinal chemistry, 17(16), 5782-5790 (2009-07-31)
Based on an established 3D pharmacophore, a series of quinoline derivatives were synthesized. The opioidergic properties of these compounds were determined by a competitive binding assay using (125)I-Dynorphine, (3)H-DAMGO and (125)I-DADLE for kappa, mu, and delta receptors, respectively. Results showed
Stefania Ferrari et al.
Journal of medicinal chemistry, 54(1), 211-221 (2010-12-04)
Folate analogue inhibitors of Leishmania major pteridine reductase (PTR1) are potential antiparasitic drug candidates for combined therapy with dihydrofolate reductase (DHFR) inhibitors. To identify new molecules with specificity for PTR1, we carried out a virtual screening of the Available Chemicals
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service