932760
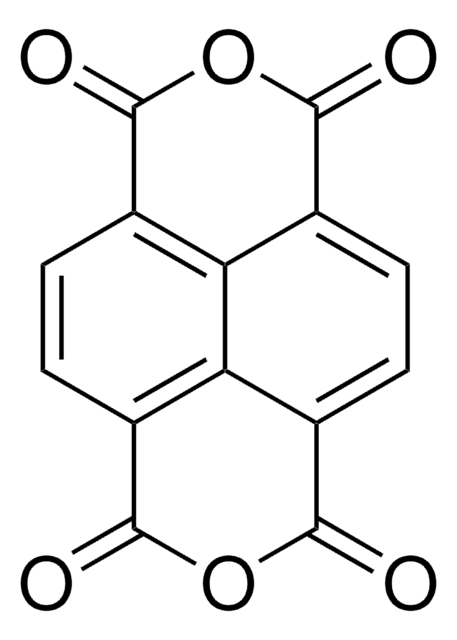
3,4,9,10-Perylenetetracarboxylic dianhydride
Synonym(s):
PTCDA, Perylenetetracarboxylic acid dianhydride, Perylene-3,4,9,10-tetracarboxylic dianhydride, Pigment Red 224
About This Item
Recommended Products
Assay
99% (sublimed)
Quality Level
mp
>300 °C
solubility
DMSO: slightly soluble
Orbital energy
HOMO 5.85 eV
LUMO 3.9 eV
InChI
1S/C24H8O6/c25-21-13-5-1-9-10-2-6-15-20-16(24(28)30-23(15)27)8-4-12(18(10)20)11-3-7-14(22(26)29-21)19(13)17(9)11/h1-8H
InChI key
CLYVDMAATCIVBF-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
The compound has attracted much interest as an organic semiconductor. It has potential applications in organic and molecular electronics, and typically used as an archetype molecular compound. PTCDA shares many properties with conventional semiconductors (with their delocalized electronic states), and insulator-like organic molecular crystals (OMCs) (with their large absorption oscillator strengths, excitonic self-trapping, polaron-assisted conduction, etc.). That is due to extremely close π-π stacking, an unusually small intermolecular distance of only 3.21 A. Among its applications, it is important to highlight the latest focus on environment monitoring, self-assembly and photocatalysis.
Storage Class Code
11 - Combustible Solids
WGK
WGK 1
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service


![9,9-Bis[4-[(4-ethenylphenyl)methoxy]phenyl]-N2,N7-di-1-naphthalenyl-N2,N7-diphenyl-9H-Fluorene-2,7-diamine ≥98% (HPLC)](/deepweb/assets/sigmaaldrich/product/structures/217/694/0386f9cf-a92c-48f3-bde7-f7a23d7cdc3a/640/0386f9cf-a92c-48f3-bde7-f7a23d7cdc3a.png)
![Benz[b]anthracene 98%](/deepweb/assets/sigmaaldrich/product/structures/197/885/3a015625-5e09-4f15-8b17-4cc285304fc7/640/3a015625-5e09-4f15-8b17-4cc285304fc7.png)