850993
2-Aminoisobutyric acid
98%, for peptide synthesis
Synonym(s):
α-Aminoisobutyric acid, 2-Methylalanine, Aib
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
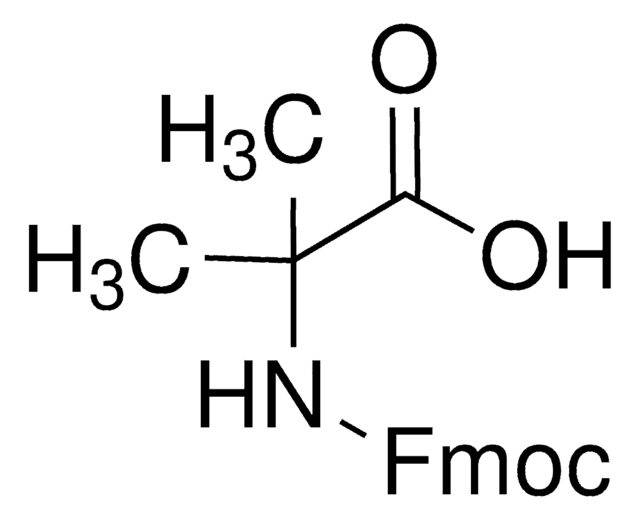
(CH3)2C(NH2)COOH
CAS Number:
Molecular Weight:
103.12
Beilstein:
506496
EC Number:
MDL number:
UNSPSC Code:
12352106
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Product Name
2-Aminoisobutyric acid, 98%
Quality Level
Assay
98%
form
solid
reaction suitability
reaction type: solution phase peptide synthesis
mp
≥300 °C
application(s)
peptide synthesis
SMILES string
CC(C)(N)C(O)=O
InChI
1S/C4H9NO2/c1-4(2,5)3(6)7/h5H2,1-2H3,(H,6,7)
InChI key
FUOOLUPWFVMBKG-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
2-Aminoisobutyric acid also known as α-aminobutyric acid, is an amino acid used in solution-phase peptide synthesis. It is a desirable building block for peptides because of its strong tendency to cause the peptide to form a helical shape.
Application
2-Aminoisobutyric acid can be used to synthesize self-assembled polypeptide nanoparticles. Incorporation of this compound into the peptide chain can prevent undesired reactions since it is di-α-substituted, and inert to C−H abstraction.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
C Lucarelli et al.
Journal of chromatography, 541(1-2), 285-296 (1991-03-22)
A procedure is described for the determination of alpha-methyldopa (MD) [L-3-(3,4-dihydroxyphenyl)-2-methylalanine], its metabolite and catecholamines in the urine and plasma of patients undergoing MD therapy, by high-performance liquid chromatography with dual working electrode coulometric detection. An efficient sample preparation procedure
Yutong Yang et al.
Membranes, 10(11) (2020-11-14)
Amine-containing mixed-matrix membranes incorporated with amino-functionalized multi-walled carbon nanotubes (AF-MWNTs) were synthesized for CO2/H2 separation based on the facilitated transport mechanism. AF-MWNTs were chosen primarily as the mechanical reinforcing filler to enhance the membrane stability. At 107 °C and 0.2-MPa
Lucia Becucci et al.
Journal of the American Chemical Society, 132(17), 6194-6204 (2010-04-16)
Four oligopeptides consisting of a sequence of alpha-aminoisobutyric acid (Aib) residues, thiolated at either the N- or C-terminus by means of a -(CH(2))(2)-SH anchor, were self-assembled on mercury, which is a substrate known to impart a high fluidity to self-assembled
Øyvind Jacobsen et al.
The Journal of organic chemistry, 76(5), 1228-1238 (2011-02-01)
Short peptides are important as lead compounds and molecular probes in drug discovery and chemical biology, but their well-known drawbacks, such as high conformational flexibility, protease lability, poor bioavailability and short half-lives in vivo, have prevented their potential from being
Anne Goj et al.
The Journal of chemical physics, 134(20), 205103-205103 (2011-06-07)
We use mixed classical/quantum simulations to study the time dependence of an excitation of a C=O vibration on a 3-10 helix of α-aminoisobutyric acid, a system which represents a test case for the formation of self-trapped vibrational excitation states on
Global Trade Item Number
| SKU | GTIN |
|---|---|
| 850993-100G | 4061832596525 |
| 850993-5KG | |
| 850993-25G | 4061833023020 |
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service