742600
Difluoromethyl phenyl sulfone
≥97%
Synonym(s):
PhSO2CF2H, [(Difluoromethyl)sulfonyl]benzene
About This Item
Recommended Products
Quality Level
Assay
≥97%
form
solid
functional group
fluoro
sulfone
SMILES string
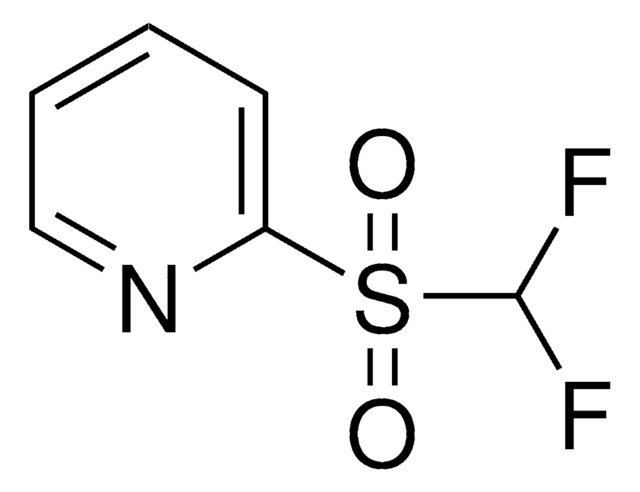
FC(F)S(=O)(=O)c1ccccc1
InChI
1S/C7H6F2O2S/c8-7(9)12(10,11)6-4-2-1-3-5-6/h1-5,7H
InChI key
LRHDNAVPELLXDL-UHFFFAOYSA-N
Application
- Reductive silylation and the preparation of trifluoro- and difluoromethylsilanes by reductive coupling of fluoromethyl sulfones, sulfoxides and sulfides with chlorosilanes
- Fluoroalkylation/chloroalkylation of α,β-enones, arynes, acetylenic ketones and other Michael acceptors
- Difluoromethylation of primary alkyl halides via nucleophilic substitution-reductive desulfonylation
Reagent used in Preparation of
- α-difluoromethyl amines via stereoselective (phenylsulfonyl)difluoromethylation of chiral sulfinyl aldimines
- Anti-difluoropropanediols via potassium tert-butoxide-catalyzed difluoromethylenation of aldehydes
- β-difluoromethylated and β-difluoromethylenated alcohols and amines by regioselective nucleophilic difluoromethylation of 1,2-cyclic sulfates and sulfamidates
- Difluoroalkenes from alkyl halides via nucleophilic substitution-elimination
- Difluoromethyl alcohol derivatives from enolizable and non-enolizable carbonyl compounds using nucleophilic phenylsulfonyldifluoromethylation-reductive desulfonylation strategy
- Fluoromethylated vicinal ethylenediamines via fluoromethylation of chiral α-aminobutanesulfinimines with (phenylsulfonyl)fluoromethanes followed by reductive desulfonylation and alcoholysis
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2
Storage Class Code
10 - Combustible liquids
WGK
WGK 3
Flash Point(F)
264.0 °F
Flash Point(C)
128.9 °C
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Related Content
The major research interests of Prof. Jinbo Hu's lab include the development of new fluorination reagents and reactions, especially the difluoromethylation, difluoromethylenation, and monofluoromethylation methods.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service