672068
Vinylphosphonic acid
≥90% (T)
Synonym(s):
Ethenephosphonic acid, Ethylenephosphonic acid, P-Ethenylphosphonic acid
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
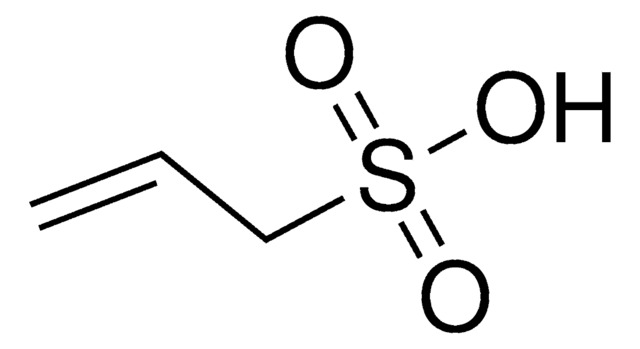
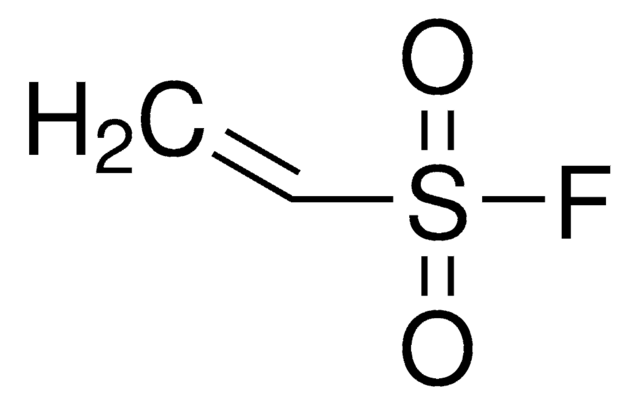
CH2=CHP(O)(OH)2
CAS Number:
Molecular Weight:
108.03
Beilstein:
1741622
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
≥90% (T)
impurities
≤7.0% water
mp
36 °C (Lit. dry VPA) (lit.)
density
1.37 g/mL at 20 °C (lit.)
SMILES string
OP(O)(=O)C=C
InChI
1S/C2H5O3P/c1-2-6(3,4)5/h2H,1H2,(H2,3,4,5)
InChI key
ZTWTYVWXUKTLCP-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
Application
Vinylphosphonic acid (VPA) can be used as a monomer unit for the synthesis of poly(vinylphosphonic acid) via free radical polymerization. It is also used to develop copolymers of VPA with acrylonitrile, N-isopropylacrylamide, styrene, vinylpyrrolidone, and acrylic and methacrylic acid. These copolymers find potential application in hydrogels, drug delivery, biomimetic mineralization, and polymer electrolyte membranes in fuel cells.
It can also be used as an organic building block to prepare (E)-styryl phosphonic acid derivatives by reacting with various aryl halides via Pd-catalyzed Heck coupling reaction.
It can also be used as an organic building block to prepare (E)-styryl phosphonic acid derivatives by reacting with various aryl halides via Pd-catalyzed Heck coupling reaction.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Met. Corr. 1 - Skin Corr. 1B
Storage Class Code
8A - Combustible corrosive hazardous materials
WGK
WGK 1
Flash Point(F)
467.6 °F
Flash Point(C)
242 °C
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Sadegh Rostamnia et al.
Journal of combinatorial chemistry, 11(1), 143-145 (2008-12-23)
Trisubstituted vinylphosphonates have been prepared via three-component reaction using phosphites, acetylenic esters, and aroyl chlorides in good yields. A variety of phosphites, activated acetylenes, and aroyl chlorides have been successfully employed in these reactions. In addition, three-component synthesis of vinylphosphonate
Zara A Doddridge et al.
Biochemistry, 42(11), 3239-3246 (2003-03-19)
We have previously reported the synthesis of vinylphosphonate-linked thymidine dimers and their incorporation into synthetic oligonucleotides to create vinylphosphonate internucleotide linkages in the DNA. Such linkages have a profound effect on DNA backbone rotational flexibility, and we have shown that
Stephan Salzinger et al.
Macromolecular rapid communications, 33(16), 1327-1345 (2012-07-11)
Recent studies have shown that poly(vinylphosphonate)s are readily accessible by rare earth metal-mediated group transfer polymerization (GTP). This article highlights the progress in this new field and advantages of GTP in comparison to classical anionic and radical polymerization approaches. Late
Xuequan Lu et al.
The Journal of organic chemistry, 74(8), 3192-3195 (2009-03-20)
The first enantioselective synthesis of chiral isosteric phosphonate analogues of FTY720 is described. One of these analogues, FTY720-(E)-vinylphosphonate (S)-5, but not its R enantiomer, elicited a potent antiapoptotic effect in intestinal epithelial cells, suggesting that it exerts its action via
Ozlem Dogan et al.
Langmuir : the ACS journal of surfaces and colloids, 22(23), 9671-9675 (2006-11-01)
In the present work, copolymers of vinylphosphonic acid and 4-vinilyimidazole (poly(4-VIm-co-VPA)) were found to be substrates favoring the precipitation of nanohydroxyapatite (HAP) crystals from stable supersaturated solutions at pH 7.4 and 37 degrees C. Deposition kinetics were studied by the
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service
![Bis[2-(methacryloyloxy)ethyl] phosphate](/deepweb/assets/sigmaaldrich/product/structures/128/336/4e7a3e38-338c-423e-95b8-70d9d1f8e121/640/4e7a3e38-338c-423e-95b8-70d9d1f8e121.png)