665355
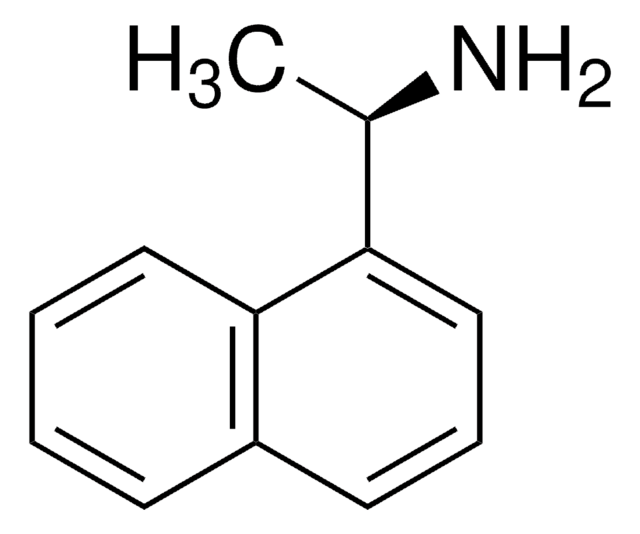
(S)-(+)-Benzyl(3,5-dioxa-4-phospha-cyclohepta[2,1-a;3,4-a′]dinaphthalen-4-yl)methylamine
97%
Synonym(s):
(+)-N-Benzyl-N-methyl-dinaphtho[2,1-d:1′,2′]dioxaphosphepin-4-amine, (11bS)
About This Item
Recommended Products
Assay
97%
form
solid
mp
155-159 °C
SMILES string
CN(Cc1ccccc1)P2Oc3ccc4ccccc4c3-c5c(O2)ccc6ccccc56
InChI
1S/C28H22NO2P/c1-29(19-20-9-3-2-4-10-20)32-30-25-17-15-21-11-5-7-13-23(21)27(25)28-24-14-8-6-12-22(24)16-18-26(28)31-32/h2-18H,19H2,1H3
InChI key
OHGGHRJGBXAKKY-UHFFFAOYSA-N
Application
Legal Information
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
Choose from one of the most recent versions:
Certificates of Analysis (COA)
Don't see the Right Version?
If you require a particular version, you can look up a specific certificate by the Lot or Batch number.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Articles
In collaboration with DSM, we are pleased to offer a range of MonoPhos™ ligands for the research market.† Feringa and co-workers have invented a diverse array of these chiral, monodentate phosphoramidites based on the privileged BINOL platform.
A diverse array of these chiral, monodentate phosphoramidites based on the privileged BINOL platform. The MonoPhos™ family has exhibited high levels of enantiocontrol in synthetic transformations ranging from metal-catalyzed asymmetric 1,4-additions of organometallic reagents to allylic alkylations to desymmetrization of meso-cycloalkene oxides.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service


![(S,S,S)-(+)-(3,5-Dioxa-4-phosphacyclohepta[2,1-a:3,4-a’]dinaphthalen-4-yl)bis(1-phenylethyl)amine 97%](/deepweb/assets/sigmaaldrich/product/structures/223/794/16c37a96-da16-488a-b3e8-7d89c47f71ee/640/16c37a96-da16-488a-b3e8-7d89c47f71ee.png)