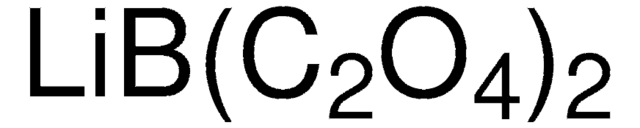544094
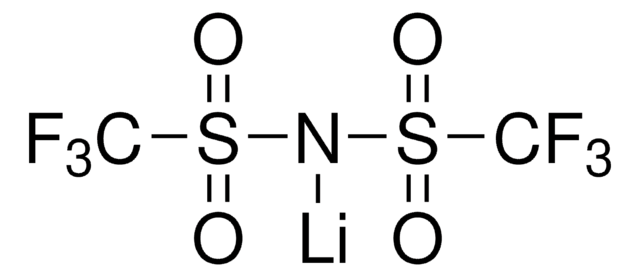
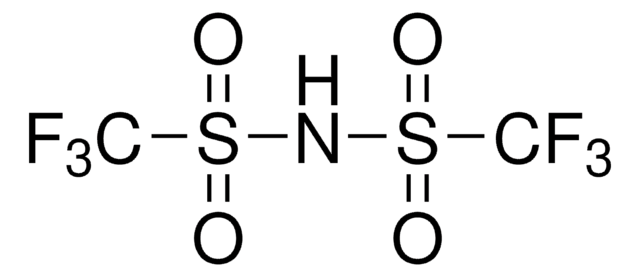
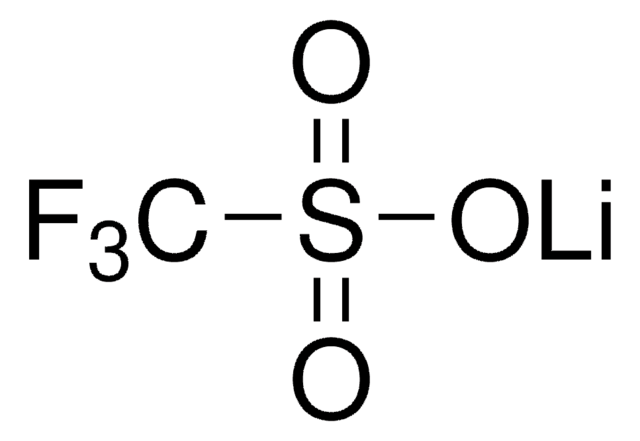
Bis(trifluoromethane)sulfonimide lithium salt
99.95% trace metals basis
Synonym(s):
Bis(trifluoromethylsulfonyl)amine lithium salt, Lithium bistrifluoromethanesulfonimidate
About This Item
Recommended Products
Quality Level
Assay
99.95% trace metals basis
mp
234-238 °C (lit.)
SMILES string
[Li]N(S(=O)(=O)C(F)(F)F)S(=O)(=O)C(F)(F)F
InChI
1S/C2F6NO4S2.Li/c3-1(4,5)14(10,11)9-15(12,13)2(6,7)8;/q-1;+1
InChI key
QSZMZKBZAYQGRS-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
LiTFSI exhibits high ionic conductivity and electrochemical stability. Used as an electrolyte additive in energy storage devices.
Application
- As ionic liquid in the preparation of gel polymer electrolytes (GPEs) using solution casting technique.
- To compose unique nonflammable electrolyte, having thermal stability beyond 200°C and a remarkably high transference number, for lithium-ion batteries.
- As electrolyte for the Li-O2 batteries.
- To compose the solid polymer electrolytes for lithium batteries.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 3 Dermal - Acute Tox. 3 Oral - Aquatic Chronic 3 - Eye Dam. 1 - Skin Corr. 1B - STOT RE 2 Oral
Target Organs
Nervous system
Storage Class Code
6.1B - Non-combustible acute toxic Cat. 1 and 2 / very toxic hazardous materials
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Articles
Ionic liquids, also called room temperature ionic liquids, are organic salts that are liquid at, or close to, room temperature.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service