All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C10H20N2O2
CAS Number:
Molecular Weight:
200.28
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
96%
mp
162-166 °C (lit.)
functional group
amine
SMILES string
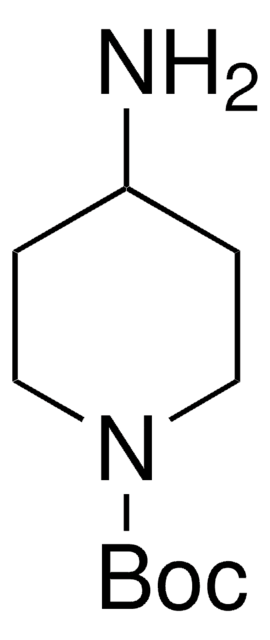
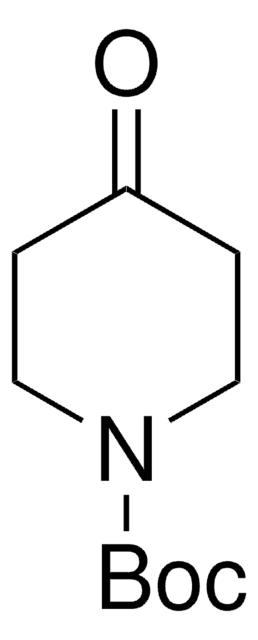
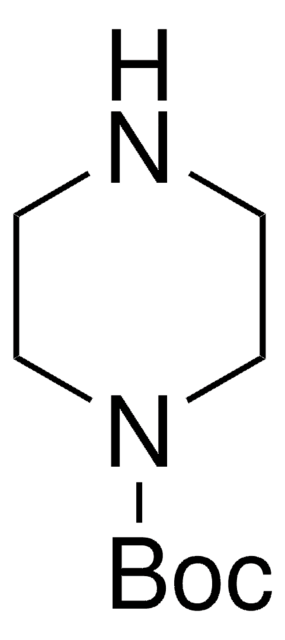
CC(C)(C)OC(=O)NC1CCNCC1
InChI
1S/C10H20N2O2/c1-10(2,3)14-9(13)12-8-4-6-11-7-5-8/h8,11H,4-7H2,1-3H3,(H,12,13)
InChI key
CKXZPVPIDOJLLM-UHFFFAOYSA-N
General description
4-(N-Boc-amino)piperidone is a piperidone derivative.
Application
4-(N-Boc-amino)piperidone may be used as a functionalization reagent for introduction of a primary and a tertiary amino group to poly(2-isopropyl-2-oxazoline.
Pharma building block.
Used in a synthesis of a piperidine-4-carboxamide CCR5 antagonist with potent anti-HIV-1 activity.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Shinichi Imamura et al.
Journal of medicinal chemistry, 49(9), 2784-2793 (2006-04-28)
We incorporated various polar groups into previously described piperidine-4-carboxamide CCR5 antagonists to improve their metabolic stability in human hepatic microsomes. Introducing a carbamoyl group into the phenyl ring of the 4-benzylpiperidine moiety afforded the less lipophilic compound 5f, which possessed
Poly (2-isopropyl-2-oxazoline)-poly (L-glutamate) block copolymers through ammonium-mediated NCA polymerization.
Meyer M and Schlaad H.
Macromolecules, 39(11), 3967-3970 (2006)
Apos Dermatakis et al.
Bioorganic & medicinal chemistry, 11(8), 1873-1881 (2003-03-28)
A series of oxindole CDK2 inhibitors was synthesized. These novel analogues have a saturated monosubstituted cyclic moiety at their C-4 position that mimics the ribofuranoside of ATP. This substitution afforded agents with increased potency relative to the parent indolinone and
Global Trade Item Number
| SKU | GTIN |
|---|---|
| 540935-1G | 4061826706213 |
| 540935-25G | 4061833398913 |
| 540935-5G | 4061833373279 |
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service