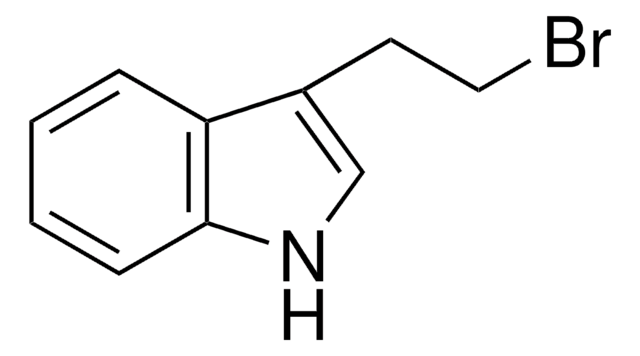
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
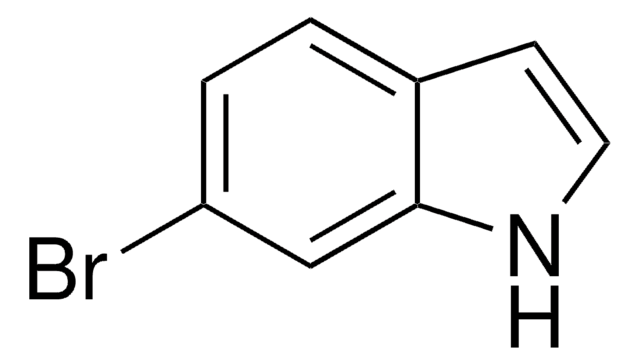
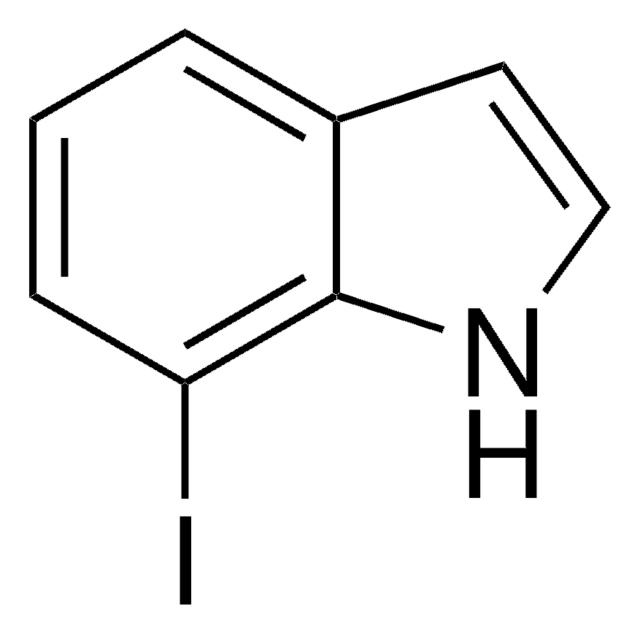
C8H6BrN
CAS Number:
Molecular Weight:
196.04
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
96%
refractive index
n20/D 1.655 (lit.)
bp
283-285 °C (lit.)
density
1.563 g/mL at 25 °C (lit.)
functional group
bromo
SMILES string
Brc1cccc2[nH]ccc12
InChI
1S/C8H6BrN/c9-7-2-1-3-8-6(7)4-5-10-8/h1-5,10H
InChI key
GRJZJFUBQYULKL-UHFFFAOYSA-N
Application
4-Bromoindole may be used to synthesize:
- clavicipitic acid, an ergot alkaloid
- 4-bromodehydrotryptophan
- 3-indolylacetonitrile derivative
- marine alkaloid dictyodendrin B
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
230.0 °F - closed cup
Flash Point(C)
110 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Optically active total synthesis of clavicipitic acid.
Yokoyama Y, et al.
The Journal of Organic Chemistry, 60(6), 1486-1487 (1995)
Metal-halogen exchange of bromoindoles. A route to substituted indoles.
Moyer MP, et al.
The Journal of Organic Chemistry, 51(26), 5106-5110 (1986)
Andrew K Pitts et al.
Angewandte Chemie (International ed. in English), 54(18), 5451-5455 (2015-02-24)
A sequential CH functionalization strategy for the synthesis of the marine alkaloid dictyodendrin B is reported. Our synthesis begins from commercially available 4-bromoindole and involves six direct functionalizations around the heteroarene core as part of a gram-scale strategy towards the natural
S Liras et al.
Journal of the American Chemical Society, 123(25), 5918-5924 (2001-06-21)
Concise syntheses of the Ergot alkaloids rugulovasine A (3a), rugulovasine B (3b), and setoclavine (2) have been completed by strategies that feature inter- and intramolecular vinylogous Mannich reactions as the key steps. Thus, the first synthesis of 3a,b commenced with
Chaitany Jayprakash Raorane et al.
Biomolecules, 10(8) (2020-08-23)
Multi-drug resistant Acinetobacter baumannii is well-known for its rapid acclimatization in hospital environments. The ability of the bacterium to endure desiccation and starvation on dry surfaces for up to a month results in outbreaks of health care-associated infections. Previously, indole
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service