520691
Platinum on silica
extent of labeling: 1 wt. % loading, granular, dry
Sign Into View Organizational & Contract Pricing
All Photos(3)
About This Item
Recommended Products
form
dry
granular
Quality Level
reaction suitability
reagent type: catalyst
core: platinum
greener alternative product characteristics
Design for Energy Efficiency
Learn more about the Principles of Green Chemistry.
sustainability
Greener Alternative Product
extent of labeling
1 wt. % loading
greener alternative category
, Enabling
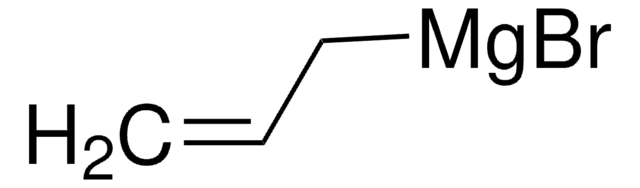
SMILES string
[Pt]
InChI
1S/Pt
InChI key
BASFCYQUMIYNBI-UHFFFAOYSA-N
Application
Platinum on silica (Pt/SiO2) can be used for a variety of applications such as:
- a substrate for the preparation of zinc oxide (ZnO) nanopillar arrays
- an electrocatalyst for methanol oxidation
- in the formation of nanospheres for catalytic hydrogen production
Other Notes
We are committed to bringing you Greener Alternative Products, which adhere to one or more of The 12 Principles of Greener Chemistry. This product has been enhanced for energy efficiency. Find details here.
Storage Class Code
13 - Non Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Photoelectrochemical hydrogen production of TiO2 passivated Pt/Si-nanowire composite photocathode
Li S, et al.
ACS Applied Materials & Interfaces, 7(33), 18560-18565 (2015)
Spatially separated ZnO nanopillar arrays on Pt/Si substrates prepared by electrochemical deposition
Lee S, et al.
The Journal of Physical Chemistry C, 111(32), 11793-11801 (2007)
Core-shell nanospheres Pt@ SiO2 for catalytic hydrogen production
Hu Y, et al.
Applied Surface Science, 341, 185-189 (2015)
Articles
Proton exchange membrane (PEM) fuel cells operate at relatively low temperatures and are composed of two electrodes and a conductive elecrolyte.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service