All Photos(3)
About This Item
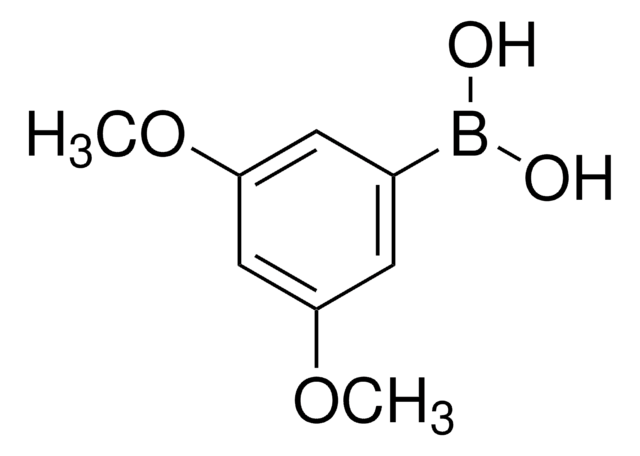
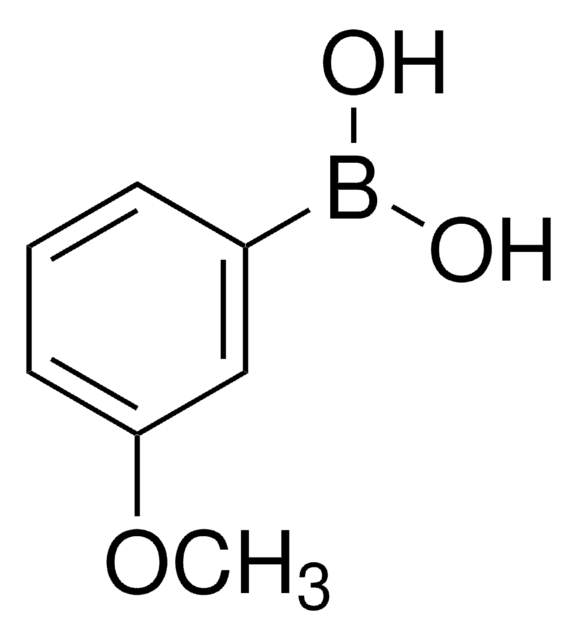
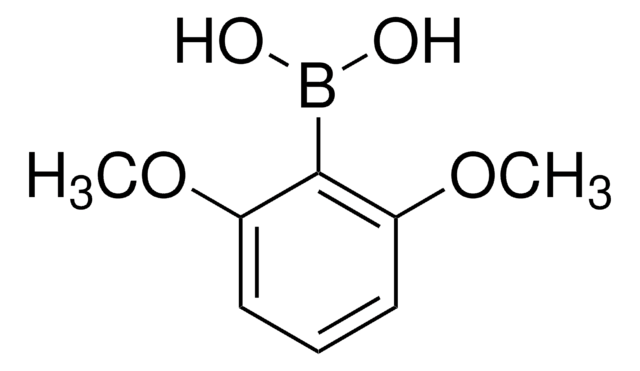
Linear Formula:
(CH3O)2C6H3B(OH)2
CAS Number:
Molecular Weight:
181.98
MDL number:
UNSPSC Code:
12352103
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
≥95.0%
mp
245-250 °C (lit.)
SMILES string
COc1ccc(cc1OC)B(O)O
InChI
1S/C8H11BO4/c1-12-7-4-3-6(9(10)11)5-8(7)13-2/h3-5,10-11H,1-2H3
InChI key
RCVDPBFUMYUKPB-UHFFFAOYSA-N
Application
3,4-Dimethoxyphenylboronic acid can be used:
- As a substrate in the cross-coupling reaction with 5,7-dichloropyrido[4,3-d]pyrimidine catalyzed by palladium.
- As a starting material for the synthesis of buflavine 1, a natural alkaloid.
- In one of the key synthetic steps for the preparation of lipidated malarial glycosylphosphatidylinositols (GPI) disaccharide.
- To prepare 3,3″,4,4″-tetramethoxy-1,1′:4′,1″-terphenyl by reacting with 1,4-dibromobenzene using Pd catalyst.
Other Notes
Contains varying amounts of anhydride
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
A Suzuki-Miyaura coupling mediated deprotection as key to the synthesis of a fully lipidated malarial GPI disaccharide
Liu X and Seeberger PH
Chemical Communications (Cambridge, England), 15, 1708-1709 (2004)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service


![[1,1′-Bis(diphenylphosphino)ferrocene]dichloropalladium(II)](/deepweb/assets/sigmaaldrich/product/structures/130/734/8846aa26-1858-458a-998d-8c306c13bf0f/640/8846aa26-1858-458a-998d-8c306c13bf0f.png)