457698
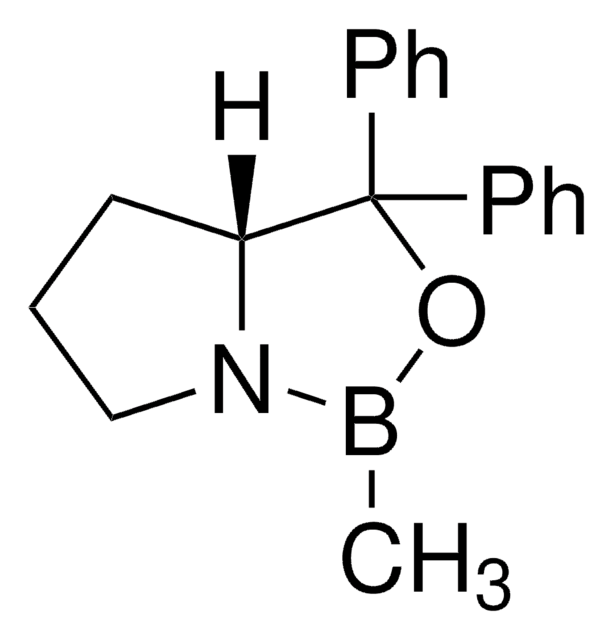
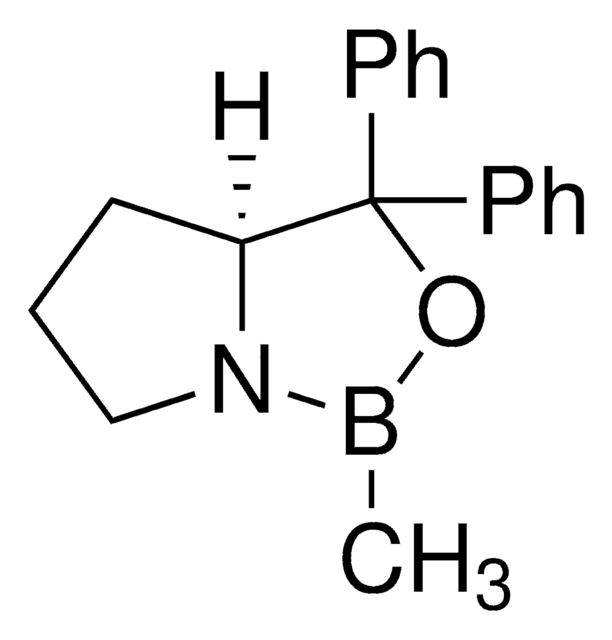
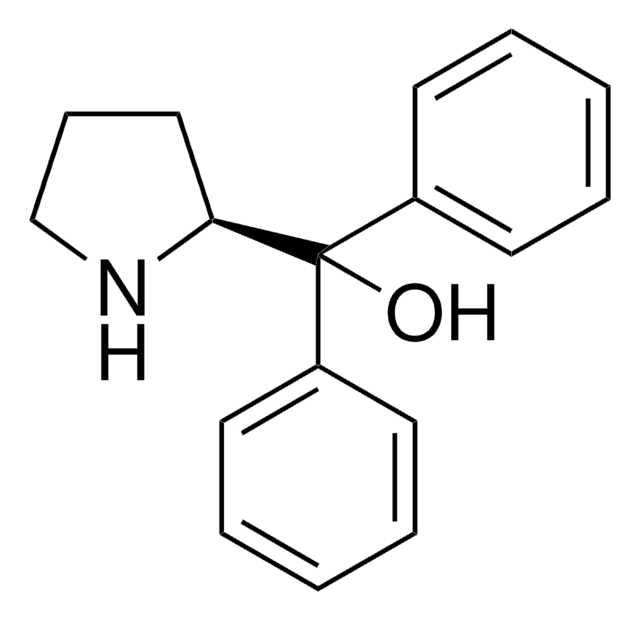
(R)-(+)-2-Methyl-CBS-oxazaborolidine solution
1 M in toluene
Synonym(s):
α,α-Diphenyl-D-prolinolmethylboronic acid cyclamide ester, (R)-1-Methyl,3,3-diphenyl-tetrahydro-pyrrolo(1,2-c)(1,3,2)oxazaborole, (R)-Tetrahydro-1-methyl-3,3-diphenyl-1H,3H-pyrrolo[1,2-c][1,3,2]oxazaborole
About This Item
Recommended Products
Quality Level
concentration
1 M in toluene
bp
111 °C
density
0.95 g/mL at 25 °C
functional group
phenyl
storage temp.
room temp
SMILES string
[H][C@]12CCCN1B(C)OC2(c3ccccc3)c4ccccc4
InChI
1S/C18H20BNO/c1-19-20-14-8-13-17(20)18(21-19,15-9-4-2-5-10-15)16-11-6-3-7-12-16/h2-7,9-12,17H,8,13-14H2,1H3/t17-/m1/s1
InChI key
VMKAFJQFKBASMU-QGZVFWFLSA-N
Looking for similar products? Visit Product Comparison Guide
Application
It may also be used in the preparation of:
- (-)-diospongin B
- (1R)-2-azido-1-(2,2-dimethyl-4H-1,3-benzodioxin-6-yl)ethanol
- (S)-α-deuteriobenzyl alcohol
- (3S,4R,5S)-1-(trimethylsilyl)-4,5-epoxyhex-1-yn-3-ol
Used in a desymmetrizing reduction leading to (S)-4-hydroxycyclohexenone.
Physical form
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Aquatic Chronic 3 - Asp. Tox. 1 - Eye Dam. 1 - Flam. Liq. 2 - Repr. 2 - Skin Irrit. 2 - STOT RE 2 - STOT SE 3
Target Organs
Central nervous system
Storage Class Code
3 - Flammable liquids
WGK
WGK 3
Flash Point(F)
39.2 °F - closed cup
Flash Point(C)
4 °C - closed cup
Personal Protective Equipment
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Articles
we are pleased to offer both enatiomers of 2-methyl-CBS-oxazaborolidine as a dry reagent, in addition to our current offerings as a 1M solution in toluene.
We are pleased to offer o-tolyl-CBS-oxazaborolidine as a 0.5 M solution in toluene for your research needs. When protonated with trifluoromethanesulfonimide, these chiral oxazaborolidines generate chiral Lewis acids, which have demonstrated great utility in the enantioselective Diels–Alder reaction.
Related Content
Our company is pleased to offer both enantiomers of 2-methyl-CBS-oxazaborolidine as a dry reagent, in addition to our current offerings as a 1 M solution in toluene.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service