All Photos(1)
About This Item
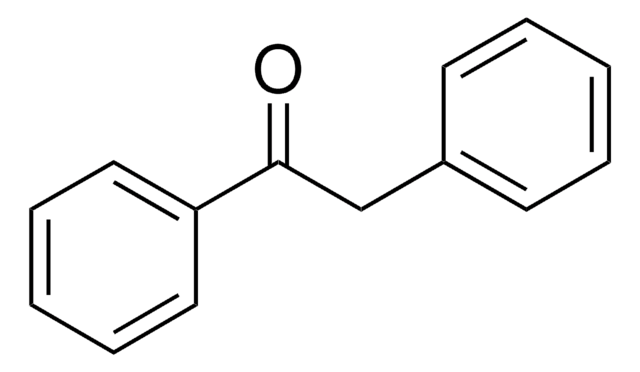
Empirical Formula (Hill Notation):
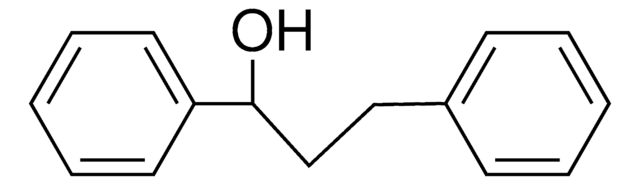
C15H14O
CAS Number:
Molecular Weight:
210.27
Beilstein:
1951697
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
≥98.0% (HPLC)
form
solid
mp
55-57 °C
functional group
hydroxyl
phenyl
storage temp.
2-8°C
SMILES string
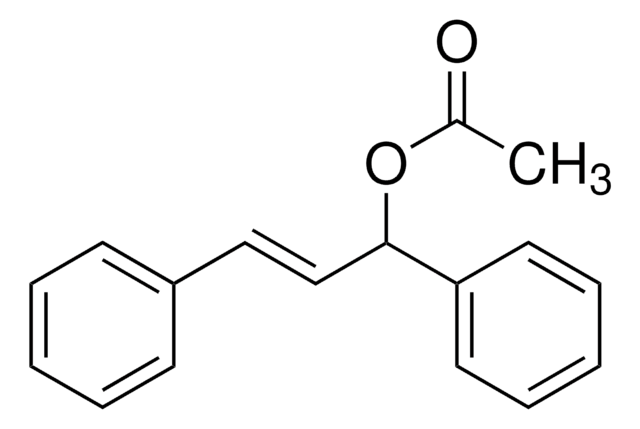
OC(\C=C\c1ccccc1)c2ccccc2
InChI
1S/C15H14O/c16-15(14-9-5-2-6-10-14)12-11-13-7-3-1-4-8-13/h1-12,15-16H/b12-11+
InChI key
ORACYDGVNJGDMI-VAWYXSNFSA-N
Looking for similar products? Visit Product Comparison Guide
General description
trans-1,3-diphenyl-2-propen-1-ol is an allylic alcohol. It has been reported to exhibit significant in vivo anti-inflammatory activity. Allylic amination of trans-1,3-diphenyl-2-propen-1-ol catalyzed by water-soluble calix[4]resorcinarene sulfonic acid has been reported.
Application
trans-1,3-diphenyl-2-propen-1-ol may be used as a model substrate to investigate the formation of substituted cyclopropanes by SiO2-ZrO2 mixed oxides catalyzed Friedel-Crafts-alkylation followed by trans-hydrogenation.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Molecular topology as a novel approach for drug discovery.
Galvez J, et al.
Expert Opinion on Drug Delivery, 7(2), 133-153 (2012)
Direct Nucleophilic SN1-Type Reactions of Alcohols.
Emer E, et al.
European Journal of Organic Chemistry, 4, 647-666 (2011)
High-Surface-Area SiO2-ZrO2 Mixed Oxides as Catalysts for the Friedel-Crafts-Type Alkylation of Arenes with Alcohols and Tandem Cyclopropanation Reactions.
Kaper H, et al.
ChemCatChem, 4(11), 1813-1818 (2012)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service