All Photos(1)
About This Item
Empirical Formula (Hill Notation):
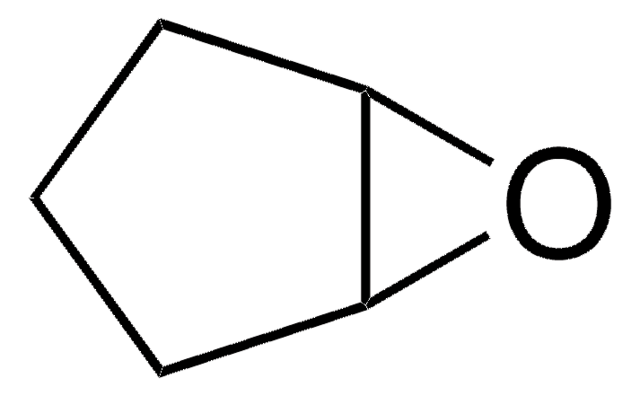
C6H8O2
CAS Number:
Molecular Weight:
112.13
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
98%
form
liquid
refractive index
n20/D 1.474 (lit.)
bp
76-78 °C/15 mmHg (lit.)
density
1.13 g/mL at 25 °C (lit.)
functional group
ether
ketone
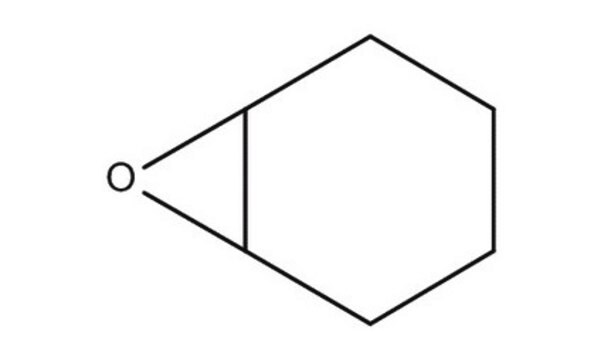
SMILES string
O=C1CCCC2OC12
InChI
1S/C6H8O2/c7-4-2-1-3-5-6(4)8-5/h5-6H,1-3H2
InChI key
QKOHEJBTNOEACF-UHFFFAOYSA-N
General description
7-Oxabicyclo[4.1.0]heptan-2-one is one of the products formed during oxidation of cyclohexene by dendritic complexes. It has been reported as anticapsin analog.
Application
7-Oxabicyclo[4.1.0]heptan-2-one was employed as substrate to investigate the substrate specificity of purified recombinant NADPH-dependent 3-quinuclidinone reductases from Microbacterium luteolum JCM 9174 for the reductive reaction of ketones.
Storage Class Code
10 - Combustible liquids
WGK
WGK 3
Flash Point(F)
204.8 °F - closed cup
Flash Point(C)
96.00 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Oxidation of cyclohexene by dendritic PAMAMSA-Mn (II) complexes.
Yang Z-W, et al.
J. Mol. Catal. A: Chem., 213(2), 169-176 (2004)
Kentaro Isotani et al.
Applied and environmental microbiology, 79(4), 1378-1384 (2012-12-25)
We used the resting-cell reaction to screen approximately 200 microorganisms for biocatalysts which reduce 3-quinuclidinone to optically pure (R)-(-)-3-quinuclidinol. Microbacterium luteolum JCM 9174 was selected as the most suitable organism. The genes encoding the protein products that reduced 3-quinuclidinone were
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service
![7-Oxabicyclo[2.2.1]heptane 98%](/deepweb/assets/sigmaaldrich/product/structures/377/935/931d29d9-08c9-492a-b42e-3f8f5a20f595/640/931d29d9-08c9-492a-b42e-3f8f5a20f595.png)