400866
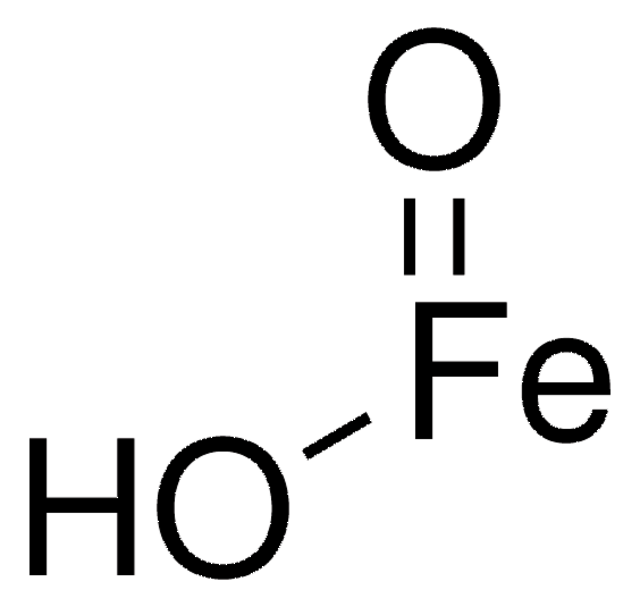
Iron(II) oxide
−10 mesh, ≥99.6% trace metals basis
Synonym(s):
Ferrous oxide, Iron monooxide
About This Item
Recommended Products
Quality Level
Assay
≥99.6% trace metals basis
form
powder
impurities
≤5% free iron
particle size
−10 mesh
density
5.7 g/mL at 25 °C (lit.)
application(s)
battery manufacturing
SMILES string
O=[Fe]
InChI
1S/Fe.O
InChI key
UQSXHKLRYXJYBZ-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
Storage Class Code
13 - Non Combustible Solids
WGK
nwg
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Articles
Magnetism and magnetic materials have been of scientific interest for over 1,000 years. More recently, fundamental investigations have focused on exploring the various types of magnetic materials and understanding the magnetic effects created by electric currents.
Magnetic materials permeate numerous daily activities in our lives. They are essential components of a diversity of products including hard drives that reliably store information on our computers, decorative magnets that keep the shopping list attached to the refrigerator door, electric bicycles that speed our commute to work, as well as wind turbines for conversion of wind energy to electrical power.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service