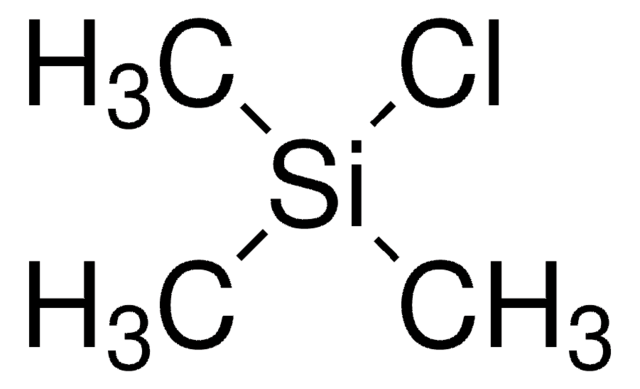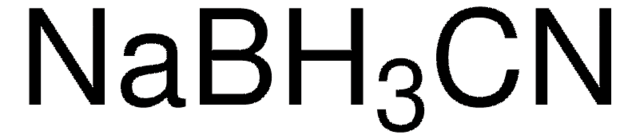384410
Chlorotrimethylsilane solution
1.0 M in THF
Synonym(s):
Trimethylchlorosilane
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
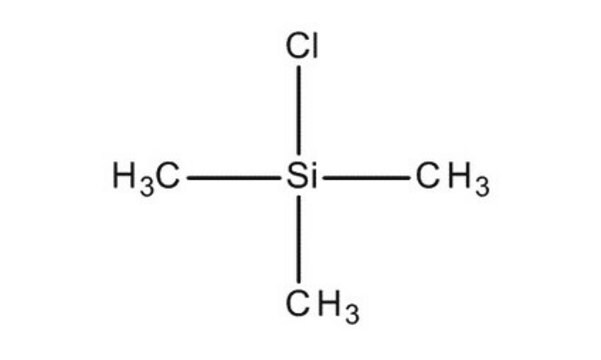
Linear Formula:
(CH3)3SiCl
CAS Number:
Molecular Weight:
108.64
Beilstein:
1209232
MDL number:
UNSPSC Code:
12352302
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
form
liquid
concentration
1.0 M in THF
density
0.88 g/mL at 25 °C
SMILES string
C[Si](C)(C)Cl
InChI
1S/C3H9ClSi/c1-5(2,3)4/h1-3H3
InChI key
IJOOHPMOJXWVHK-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
Chlorotrimethylsilane (TMSCl) is a silylating agent employed in the silylation of alcohols, amines, carboxylic acids, thiols, and other functional groups. Silyl group serves as a protecting group for these original functional groups.
Application
Chlorotrimethylsilane (TMSCl) can be used as a reagent:
- To synthesize allenylsilane via carbolithiation of conjugated enynes with aryllithium.
- In the reductive coupling of aldehydes and ketones to produce corresponding 1,2 diols in the presence of zirconocene dichloride and magnesium.
- To prepare 4,4′-bis(trimethylsilyl)bicyclohexyl-2,2′-diene from 1,3-cyclohexadiene by reductive disilylation reaction.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral - Carc. 2 - Flam. Liq. 2 - Skin Corr. 1A - STOT SE 3
Target Organs
Respiratory system
Supplementary Hazards
Storage Class Code
3 - Flammable liquids
WGK
WGK 1
Flash Point(F)
-4.0 °F - closed cup
Flash Point(C)
-20 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Zirconocene dichloride-catalyzed pinacol coupling of aromatic aldehydes and ketones
Lakshmi Kantam M, et al.
Synthetic Communications, 36(10), 1437-1445 (2006)
Carbolithiation of conjugated enynes with aryllithiums in microflow system and applications to synthesis of allenylsilanes
Tomida Y, et al.
Organic Letters, 11(16), 3614-3617 (2009)
Ainara Sistiaga et al.
PloS one, 9(6), e101045-e101045 (2014-06-26)
Neanderthal dietary reconstructions have, to date, been based on indirect evidence and may underestimate the significance of plants as a food source. While zooarchaeological and stable isotope data have conveyed an image of Neanderthals as largely carnivorous, studies on dental
Mark Sundberg et al.
Langmuir : the ACS journal of surfaces and colloids, 22(17), 7302-7312 (2006-08-09)
We have previously described the efficient guidance and unidirectional sliding of actin filaments along nanosized tracks with adsorbed heavy meromyosin (HMM; myosin II motor fragment). In those experiments, the tracks were functionalized with trimethylchlorosilane (TMCS) by chemical vapor deposition (CVD)
Malin Persson et al.
Langmuir : the ACS journal of surfaces and colloids, 26(12), 9927-9936 (2010-03-27)
In the in vitro motility assay, actin filaments are propelled by surface-adsorbed myosin motors, or rather, myosin motor fragments such as heavy meromyosin (HMM). Recently, efforts have been made to develop actomyosin powered nanodevices on the basis of this assay
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service