All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C7H5ClN2
CAS Number:
Molecular Weight:
152.58
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
97%
form
powder
mp
149 °C (subl.) (lit.)
SMILES string
Clc1n[nH]c2ccccc12
InChI
1S/C7H5ClN2/c8-7-5-3-1-2-4-6(5)9-10-7/h1-4H,(H,9,10)
InChI key
QPHAGNNWDZSKJH-UHFFFAOYSA-N
Related Categories
General description
3-Chloroindazole is an indazole bearing an electron withdrawing substituent.
Application
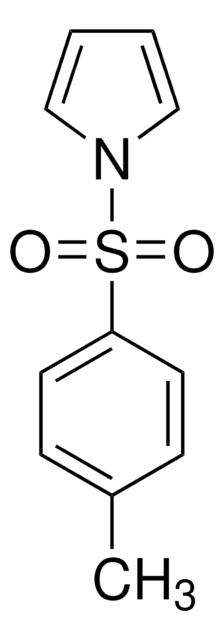
3-Chloroindazole was used in the preparation of 3-chloro-1-(4′-methylphenyl)-indazole.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Mikhail A Kinzhalov et al.
Dalton transactions (Cambridge, England : 2003), 42(29), 10394-10397 (2013-06-21)
A reaction between equimolar amounts of cis-[PdCl2(CNCy)2] (1) and indazole (HInd, 2) or 5-methylindazole (HInd(Me), 3) proceeded in refluxing CHCl3 for ca. 6 h affording the aminocarbene species cis-[PdCl2{C(Ind)=N(H)Cy}(CNCy)] (4) or cis-[PdCl2{C(Ind(Me))=N(H)Cy}(CNCy)] (5) in good (72-83%) isolated yields. Complexes 4
Jon C Antilla et al.
The Journal of organic chemistry, 69(17), 5578-5587 (2004-08-17)
This paper details the copper-catalyzed N-arylation of pi-excessive nitrogen heterocycles. The coupling of either aryl iodides or aryl bromides with common nitrogen heterocycles (pyrroles, pyrazoles, indazoles, imidazoles, and triazoles) was successfully performed in good yield with catalysts derived from diamine
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service