All Photos(1)
About This Item
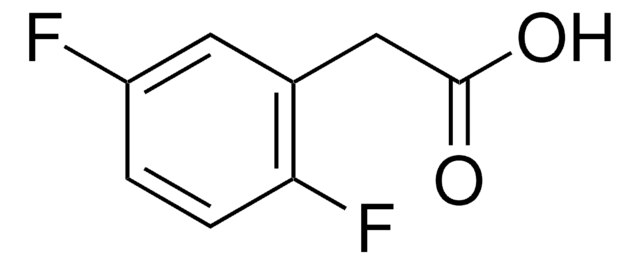
Linear Formula:
F2C6H3CH2CO2H
CAS Number:
Molecular Weight:
172.13
Beilstein:
2577768
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
98%
form
solid
mp
46-50 °C (lit.)
functional group
carboxylic acid
fluoro
SMILES string
OC(=O)Cc1ccc(F)c(F)c1
InChI
1S/C8H6F2O2/c9-6-2-1-5(3-7(6)10)4-8(11)12/h1-3H,4H2,(H,11,12)
InChI key
YCAKYFIYUHHCKW-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
The separation of five positional isomers from 3,4-difluorophenylacetic acid has been investigated using normal- and reversed-phase HPLC, capillary zone electrophoresis, gas chromatography and supercritical fluid chromatography.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
235.4 °F - closed cup
Flash Point(C)
113 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
L Zhou et al.
Journal of chromatography. A, 866(2), 281-292 (2000-02-12)
The separation of five positional isomers from 3,4-difluorophenylacetic acid was investigated using normal- and reversed-phase high-performance liquid chromatography, capillary zone electrophoresis, gas chromatography and supercritical fluid chromatography. Operating parameters of each technique, such as temperature, type of stationary phase, mobile
Sebastian Demkowicz et al.
Chemical biology & drug design, 87(2), 233-238 (2015-08-19)
In the present work, we report the initial results of our study on a series of 3-phenylcoumarin sulfamate-based compounds containing C-F bonds as novel inhibitors of steroid sulfatase. The new compounds are potent steroid sulfatase inhibitors, possessing more than 10
Mateusz Daśko et al.
European journal of medicinal chemistry, 128, 79-87 (2017-02-06)
In the present work, we report convenient methods for the synthesis of 3-(4-aminophenyl)-coumarin-7-O-sulfamate derivatives N-acylated with fluorinated analogues of benzoic or phenylacetic acid as steroid sulfatase (STS) inhibitors. The design of these potential STS inhibitors was supported by molecular modeling
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service