281255
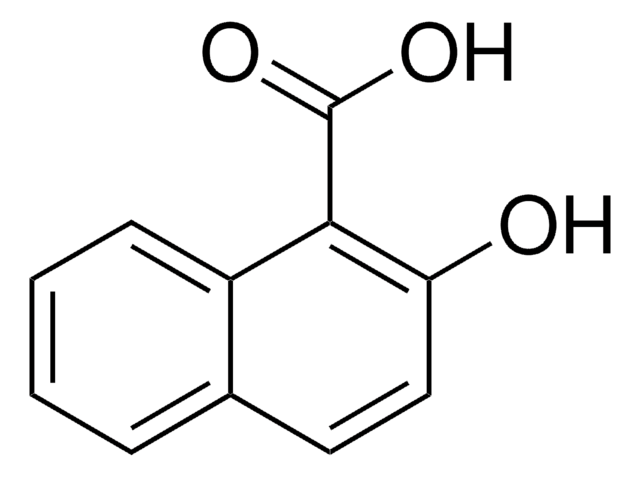
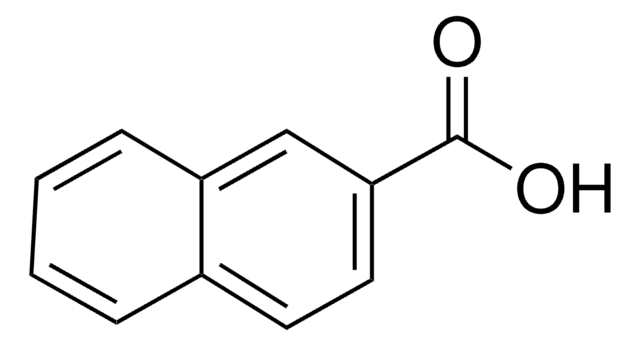
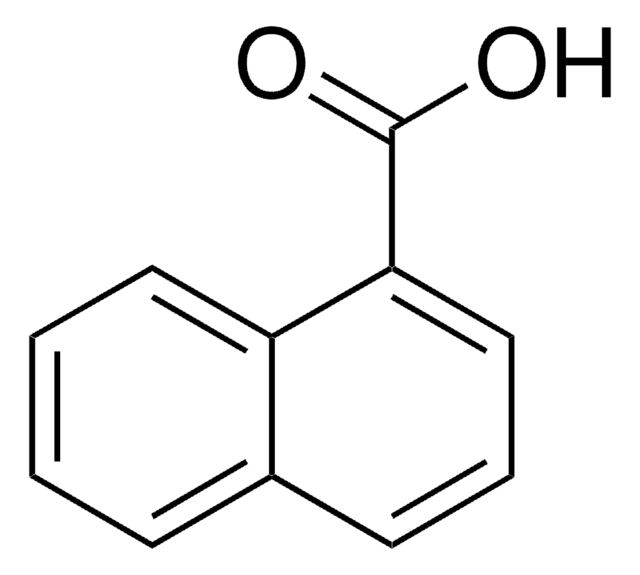
1,4-Dihydroxy-2-naphthoic acid
97%
Synonym(s):
1,4-Dihydroxy-2-carboxy naphthoic acid, 1,4-Dihydroxy-2-naphthalenecarboxylic acid, 1,4-Dihydroxy-2-naphthoate
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Linear Formula:
(HO)2C10H5CO2H
CAS Number:
Molecular Weight:
204.18
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
97%
mp
220 °C (dec.) (lit.)
functional group
carboxylic acid
SMILES string
OC(=O)c1cc(O)c2ccccc2c1O
InChI
1S/C11H8O4/c12-9-5-8(11(14)15)10(13)7-4-2-1-3-6(7)9/h1-5,12-13H,(H,14,15)
InChI key
VOJUXHHACRXLTD-UHFFFAOYSA-N
Related Categories
General description
1,4-Dihydroxy-2-naphthoic acid from Propionibacterium freudenreichii is known to promote the proliferation of Bifidobacterium. It has potential therapeutic application for psoriasis treatment.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
D J Shaw et al.
Journal of bacteriology, 152(3), 1132-1137 (1982-12-01)
Four independent menaquinone (vitamin K(2))-deficient mutants of Escherichia coli, blocked in the conversion of o-succinylbenzoate (OSB) to 1,4-dihydroxy-2-naphthoate (DHNA), were found to represent two distinct classes. Enzymatic complementation was observed when a cell-free extract of one mutant was mixed with
M Matsubara et al.
Osteoporosis international : a journal established as result of cooperation between the European Foundation for Osteoporosis and the National Osteoporosis Foundation of the USA, 21(8), 1437-1447 (2009-10-09)
The main component of the metabolic by-products of fermentation by Propionibacterium freudenreichii ET-3 is 1,4-dihydroxy-2-naphthoic acid (DHNA), which has a naphthoquinone skeleton, as in vitamin K2. This study showed that DHNA improved bone mass reduction with osteoporosis model mice caused
Alexey P Kostikov et al.
The Journal of organic chemistry, 72(24), 9190-9194 (2007-10-26)
Irradiation of alcohols, phenols, and carboxylic acids "caged" with the 2,5-dihydroxybenzyl group or its naphthalene analogue results in the efficient release of the substrate. The initial byproduct of the photoreaction, 4-hydroxyquinone-2-methide, undergoes rapid tautomerization into methyl p-quinone. The UV spectrum
K Suvarna et al.
Journal of bacteriology, 180(10), 2782-2787 (1998-06-06)
A key reaction in the biosynthesis of menaquinone involves the conversion of the soluble bicyclic naphthalenoid compound 1, 4-dihydroxy-2-naphthoic acid (DHNA) to the membrane-bound demethylmenaquinone. The enzyme catalyzing this reaction, DHNA-octaprenyltransferase, attaches a 40-carbon side chain to DHNA. The menA
Junichi Sakai et al.
Bioorganic & medicinal chemistry, 16(9), 4854-4859 (2008-04-05)
Ac-DNLD-CHO is a novel caspase-3 specific peptide inhibitor that was rationally designed by our computational strategy. The specificity was shown to be due to the specific interaction of NLD moiety with the active site of caspase-3 on the basis of
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service