270148
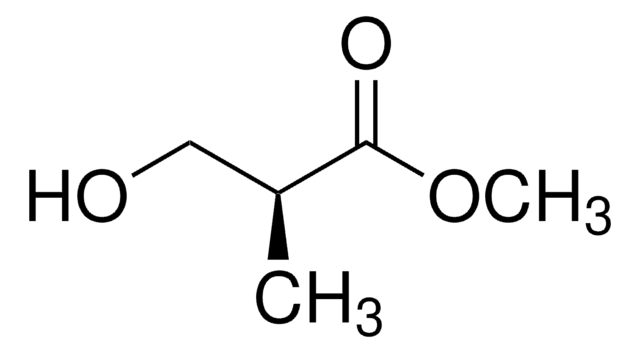
Methyl (R)-(−)-3-hydroxy-2-methylpropionate
99%
Synonym(s):
(−)-Methyl D-β-hydroxyisobutyrate, (R)-(−)-3-Hydroxy-2-methylpropionic acid methyl ester
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
HOCH2CH(CH3)CO2CH3
CAS Number:
Molecular Weight:
118.13
Beilstein:
3587507
MDL number:
UNSPSC Code:
12352108
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
99%
form
liquid
optical activity
[α]19/D −26°, c = 4 in methanol
optical purity
ee: 99% (GLC)
refractive index
n20/D 1.425 (lit.)
bp
76-77 °C/12 mmHg (lit.)
density
1.066 g/mL at 25 °C (lit.)
SMILES string
COC(=O)[C@H](C)CO
InChI
1S/C5H10O3/c1-4(3-6)5(7)8-2/h4,6H,3H2,1-2H3/t4-/m1/s1
InChI key
ATCCIZURPPEVIZ-SCSAIBSYSA-N
Related Categories
Application
Methyl (R)-(-)-3-hydroxy-2-methylpropionate may be used in the preparation of (S)-(4-benzyloxy-3-methylbut-1-ynyl)triethylsilane. It may also be used as a starting material in the total synthesis of (-)-discodermolide, (-)-stemoamide and (-)-stemospironine.
The (R)- and (S)-isomers are bifunctional building blocks for the synthesis of a wide variety of optically active molecules.
Storage Class Code
3 - Flammable liquids
WGK
WGK 3
Flash Point(F)
176.0 °F - closed cup
Flash Point(C)
80 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Total Synthesis of (-)-Stemospironine.
Williams DR, et al.
Organic Letters, 3(17), 2721-2724 (2001)
Tetrahedron Letters, 34, 5939-5939 (1993)
Distinct binding and cellular properties of synthetic (+)-and (-)-discodermolides.
Hung DT, et al.
Chemistry & Biology, 1(1), 67-71 (1994)
Recent progress in the chemistry of the Stemona alkaloids.
Pilli RA and de Oliveira MDCF.
Natural Product Reports, 17(1), 117-127 (2000)
Journal of the Chemical Society. Perkin Transactions 1, 759-759 (1993)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service